当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of the selective and nanomolar inhibitor of DPP-4 more potent than sitagliptin by structure-guided rational design
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.ejmech.2024.116834 Bushra Mobeen 1 , Muhammad Shah 1 , Hafiz Muzzammel Rehman 2 , Muhammad Saeed Jan 3 , Umer Rashid 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.ejmech.2024.116834 Bushra Mobeen 1 , Muhammad Shah 1 , Hafiz Muzzammel Rehman 2 , Muhammad Saeed Jan 3 , Umer Rashid 1
Affiliation
|
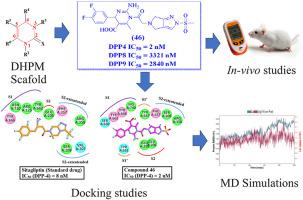
Various therapeutic targets and approaches are commonly employed in the management of Type 2 Diabetes. These encompass diverse groups of drugs that target different mechanisms involved in glucose regulation. Inhibition of the DPP-4 enzyme has been proven an excellent target for antidiabetic drug design. Our previous work on discovering multitarget antidiabetic drugs led to the identification of a gallic acid-thiazolidinedione hybrid as a potent DPP4 inhibitor (IC50 = 36 nM). In current research, our efforts resulted in a new dihydropyrimidine-based scaffold with enhanced DPP4 inhibition potential. After virtual evaluation, the designed molecules with excellent interaction patterns and binding energy values were synthesized in the wet laboratory. The inhibition potential of synthesized compounds was assessed against the DPP-4 enzyme. Compound 46 with single digit IC50 value 2 nM exhibited 4-fold and 18-fold higher activity than Sitagliptin and our previously reported hybrid respectively. Moreover, compounds 46 , 47 and 50 have shown manyfold selectivity against DPP8 and DPP9. Further pretreatment with compounds 43 , 45 –47 and 50 (at doses of 10 and 20 mg/kg) in OGTT conducted on rats resulted in a significant decrease in the serum glucose levels compared to the control group. In the long-term STZ-induced diabetic rats, tested compound 50 performed similarly to the reference drug. Molecular dynamics simulations and in-silico molecular docking studies were employed to elucidate the time-dependent interactions of inhibitors within the active sites of DPP4. The compounds examined in this work might serve as a possible lead in the development of effective diabetic mellitus treatments.
中文翻译:

通过结构引导的理性设计发现比西格列汀更有效的 DPP-4 选择性和纳摩尔抑制剂
2 型糖尿病的管理通常采用各种治疗靶点和方法。这些包括针对葡萄糖调节涉及的不同机制的不同药物组。抑制 DPP-4 酶已被证明是抗糖尿病药物设计的极好靶标。我们之前关于发现多靶点抗糖尿病药物的工作导致确定没食子酸-噻唑烷二酮杂交体是一种有效的 DPP4 抑制剂 (IC50 = 36 nM)。在目前的研究中,我们的努力产生了一种新的基于二氢嘧啶的支架,具有增强的 DPP4 抑制潜力。经过虚拟评估,在湿实验室中合成了设计出具有优异相互作用模式和结合能值的分子。根据 DPP-4 酶评估合成化合物的抑制潜力。IC50 值为 2 nM 的化合物 46 的活性分别比西格列汀和我们之前报道的杂交种高 4 倍和 18 倍。此外,化合物 46、47 和 50 对 DPP8 和 DPP9 显示出许多倍的选择性。与对照组相比,在 OGTT 中用化合物 43、45-47 和 50(剂量为 10 和 20 mg/kg)进行进一步预处理,导致血糖水平显着降低。在长期 STZ 诱导的糖尿病大鼠中,测试化合物 50 的表现与参考药物相似。采用分子动力学模拟和计算机分子对接研究来阐明 DPP4 活性位点内抑制剂的时间依赖性相互作用。这项工作中检查的化合物可能作为开发有效糖尿病治疗的可能线索。
更新日期:2024-09-05
中文翻译:

通过结构引导的理性设计发现比西格列汀更有效的 DPP-4 选择性和纳摩尔抑制剂
2 型糖尿病的管理通常采用各种治疗靶点和方法。这些包括针对葡萄糖调节涉及的不同机制的不同药物组。抑制 DPP-4 酶已被证明是抗糖尿病药物设计的极好靶标。我们之前关于发现多靶点抗糖尿病药物的工作导致确定没食子酸-噻唑烷二酮杂交体是一种有效的 DPP4 抑制剂 (IC50 = 36 nM)。在目前的研究中,我们的努力产生了一种新的基于二氢嘧啶的支架,具有增强的 DPP4 抑制潜力。经过虚拟评估,在湿实验室中合成了设计出具有优异相互作用模式和结合能值的分子。根据 DPP-4 酶评估合成化合物的抑制潜力。IC50 值为 2 nM 的化合物 46 的活性分别比西格列汀和我们之前报道的杂交种高 4 倍和 18 倍。此外,化合物 46、47 和 50 对 DPP8 和 DPP9 显示出许多倍的选择性。与对照组相比,在 OGTT 中用化合物 43、45-47 和 50(剂量为 10 和 20 mg/kg)进行进一步预处理,导致血糖水平显着降低。在长期 STZ 诱导的糖尿病大鼠中,测试化合物 50 的表现与参考药物相似。采用分子动力学模拟和计算机分子对接研究来阐明 DPP4 活性位点内抑制剂的时间依赖性相互作用。这项工作中检查的化合物可能作为开发有效糖尿病治疗的可能线索。































 京公网安备 11010802027423号
京公网安备 11010802027423号