当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Benchtop NMR-Based In-Line Analysis of Diastereoselective Enzymatic α-Amino Acid Synthesis: Quantification and Validation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.oprd.4c00076 Luca F. Schmidt 1 , Logia Jolly 2 , Leon Hennecke 1 , Fernando Lopez Haro 1 , Harald Gröger 2 , Andreas Liese 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.oprd.4c00076 Luca F. Schmidt 1 , Logia Jolly 2 , Leon Hennecke 1 , Fernando Lopez Haro 1 , Harald Gröger 2 , Andreas Liese 1
Affiliation
|
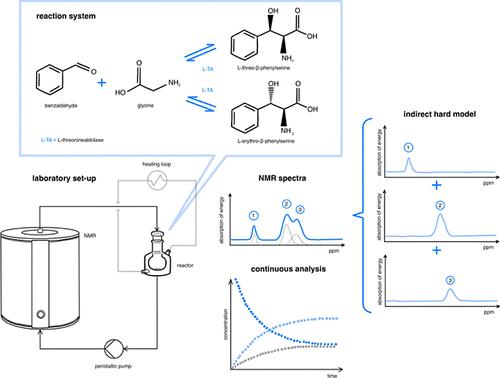
This study investigates the application of a commercial low-field benchtop NMR for real-time monitoring of enzymatically catalyzed reactions, focusing on the diastereoselectivity of the threonine aldolase-catalyzed stereoselective aldol reaction between glycine and benzaldehyde. Despite the signal overlap inherent in the weak electromagnetic field of the benchtop NMR system, a complemental hard modeling (CHM) approach effectively differentiates between diastereomers, enabling the determination of enzymatic diastereoselectivity and the transition from kinetic to thermodynamic control. In particular, the achievement of thermodynamic equilibrium in the enzymatic aldol reaction is observed for the first time using in-line methods, occurring at 30% benzaldehyde conversion after 2 h. In-line NMR analysis reveals a diastereomeric excess of 37:63 (erythro/threo), which closely aligns with off-line measurements via GC and HPLC (36:64). This determination of diastereomers using CHM enhances the efficiency of in-line monitoring in enzymatic reactions, promising significant advancements in pharmaceutical process development. Overall, the study underscores the utility of benchtop NMR systems for in-line analysis of enzymatic reactions, offering insights into reaction mechanisms, selectivity, and equilibrium dynamics, thereby facilitating more efficient process optimization in the area of fine chemicals.
中文翻译:

基于台式 NMR 的非对映选择性酶 α-氨基酸合成在线分析:定量和验证
本研究研究了商用低场台式 NMR 在酶催化反应实时监测中的应用,重点关注甘氨酸和苯甲醛之间苏氨酸醛酶催化的立体选择性羟醛反应的非对映选择性。尽管台式核磁共振系统的弱电磁场固有信号重叠,但互补硬建模 (CHM) 方法有效地区分了非对映异构体,从而能够确定酶促非对映选择性以及从动力学到热力学控制的过渡。特别是,使用在线方法首次观察到酶促羟醛反应中热力学平衡的实现,2 小时后苯甲醛转化率为 30%。在线 NMR 分析显示非对映异构体过量为 37:63(erythro/threo),这与通过 GC 和 HPLC 进行离线测量 (36:64) 密切相关。使用 CHM 测定非对映异构体提高了酶促反应中在线监测的效率,有望在制药工艺开发方面取得重大进展。总体而言,该研究强调了台式 NMR 系统在酶促反应在线分析中的实用性,提供了对反应机理、选择性和平衡动力学的见解,从而促进了精细化工领域更高效的工艺优化。
更新日期:2024-09-13
中文翻译:

基于台式 NMR 的非对映选择性酶 α-氨基酸合成在线分析:定量和验证
本研究研究了商用低场台式 NMR 在酶催化反应实时监测中的应用,重点关注甘氨酸和苯甲醛之间苏氨酸醛酶催化的立体选择性羟醛反应的非对映选择性。尽管台式核磁共振系统的弱电磁场固有信号重叠,但互补硬建模 (CHM) 方法有效地区分了非对映异构体,从而能够确定酶促非对映选择性以及从动力学到热力学控制的过渡。特别是,使用在线方法首次观察到酶促羟醛反应中热力学平衡的实现,2 小时后苯甲醛转化率为 30%。在线 NMR 分析显示非对映异构体过量为 37:63(erythro/threo),这与通过 GC 和 HPLC 进行离线测量 (36:64) 密切相关。使用 CHM 测定非对映异构体提高了酶促反应中在线监测的效率,有望在制药工艺开发方面取得重大进展。总体而言,该研究强调了台式 NMR 系统在酶促反应在线分析中的实用性,提供了对反应机理、选择性和平衡动力学的见解,从而促进了精细化工领域更高效的工艺优化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号