当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Practical Telescoped Process to Prepare (P)-7-(2-Amino-6-fluorophenyl)-4-hydroxy-6-(trifluoromethyl)pyrido[3,4-d]pyrimidin-8(7H)-one: a Key Intermediate of KRASG12C Inhibitor GH35
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.oprd.4c00279 Gaolei Zuo 1 , Haojie Xu 1, 2, 3 , Yaobin Zhang 1 , Zhi Liu 1 , Jinyue Tu 1 , Donghui Gou 1 , Peng Fu 4 , Haifeng Huang 5 , Jianhua Ren 5 , Yuanyuan Hu 5 , Feng Liu 2, 3 , Jie Jack Li 1 , Guiping Zhang 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.oprd.4c00279 Gaolei Zuo 1 , Haojie Xu 1, 2, 3 , Yaobin Zhang 1 , Zhi Liu 1 , Jinyue Tu 1 , Donghui Gou 1 , Peng Fu 4 , Haifeng Huang 5 , Jianhua Ren 5 , Yuanyuan Hu 5 , Feng Liu 2, 3 , Jie Jack Li 1 , Guiping Zhang 1
Affiliation
|
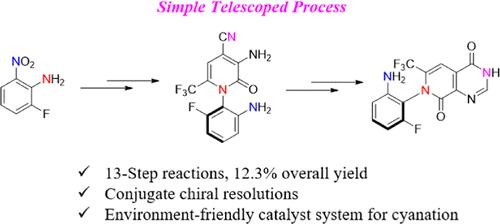
GH35 is a potent irreversible covalent inhibitor of KRASG12C that is currently undergoing phase I clinical trials for the treatment of patients with advanced solid tumors. Herein, we describe an efficient and cost-effective telescoped process to produce an atropisomeric intermediate, GH35-RSM, for the synthesis of GH35. Iterative optimization based on medicinal chemistry synthetic route resulted in significant improvement of several key reactions, such as two-step chiral resolution affording highly pure atropisomer (P)-methyl 1-(2-fluoro-6-nitrophenyl)-2-oxo-6-(trifluoromethyl)-1,2-dihydropyridine-3-carboxylate (5-P) in 99.9% e.e. and 35% yield and introduction of a nitrile group into (P)-3-amino-1-(2-amino-6-fluorophenyl)-2-oxo-6-(trifluoromethyl)-1,2-dihydropyridine-4-carbonitrile (17) by use of an environment-friendly catalyst system identified by high throughput screening. The telescoped six-step process was robustly performed to provide hundreds of kilograms of GH35-RSM in plants to support clinical studies.
中文翻译:

制备 (P)-7-(2-氨基-6-氟苯基)-4-羟基-6-(三氟甲基)嘧啶-8(7H)-酮的实用望远镜工艺的开发:KRASG12C抑制剂 GH35 的关键中间体
GH35 是一种有效的不可逆 KRASG12C 共价抑制剂,目前正在进行 I 期临床试验,用于治疗晚期实体瘤患者。在本文中,我们描述了一种高效且具有成本效益的望远镜工艺,用于生产用于合成 GH35 的空斜异构体中间体 GH35-RSM。基于药物化学合成路线的迭代优化导致几个关键反应的显著改进,例如两步手性分离,在 99.9% e 中提供高纯度的阿托溴异构体 (P)-甲基 1-(2-氟-6-硝基苯基)-2-氧代-6-(三氟甲基)-1,2-二氢吡啶-3-羧酸盐 (5-P)。e. 使用通过高通量筛选鉴定的环保催化剂系统,将腈基引入 (P)-3-氨基-1-(2-氨基-6-氟苯基)-2-氧代-6-(三氟甲基)-1,2-二氢吡啶-4-碳腈 (17) 中。望远镜式六步工艺稳健执行,在植物中提供数百公斤的 GH35-RSM 以支持临床研究。
更新日期:2024-09-13
中文翻译:

制备 (P)-7-(2-氨基-6-氟苯基)-4-羟基-6-(三氟甲基)嘧啶-8(7H)-酮的实用望远镜工艺的开发:KRASG12C抑制剂 GH35 的关键中间体
GH35 是一种有效的不可逆 KRASG12C 共价抑制剂,目前正在进行 I 期临床试验,用于治疗晚期实体瘤患者。在本文中,我们描述了一种高效且具有成本效益的望远镜工艺,用于生产用于合成 GH35 的空斜异构体中间体 GH35-RSM。基于药物化学合成路线的迭代优化导致几个关键反应的显著改进,例如两步手性分离,在 99.9% e 中提供高纯度的阿托溴异构体 (P)-甲基 1-(2-氟-6-硝基苯基)-2-氧代-6-(三氟甲基)-1,2-二氢吡啶-3-羧酸盐 (5-P)。e. 使用通过高通量筛选鉴定的环保催化剂系统,将腈基引入 (P)-3-氨基-1-(2-氨基-6-氟苯基)-2-氧代-6-(三氟甲基)-1,2-二氢吡啶-4-碳腈 (17) 中。望远镜式六步工艺稳健执行,在植物中提供数百公斤的 GH35-RSM 以支持临床研究。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号