当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Process Development for the First GMP Synthesis of SGD-9501-TFA, Part 2: Synthesis of the Payload, Linker, and Drug Linker
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.oprd.4c00318 Katherine Payne 1 , Jeremy C. Tran 1 , Brooke Gill 1 , William Guy 1 , Caitlyn McNichol 2 , Kareem Bdeir 1 , Malcolm Reider 1 , Andrea Stair 1 , Lorenzo Pontini 3 , Gabriele Cerai 3 , Jacopo Roletto 3 , Aaron M. Whittaker 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.oprd.4c00318 Katherine Payne 1 , Jeremy C. Tran 1 , Brooke Gill 1 , William Guy 1 , Caitlyn McNichol 2 , Kareem Bdeir 1 , Malcolm Reider 1 , Andrea Stair 1 , Lorenzo Pontini 3 , Gabriele Cerai 3 , Jacopo Roletto 3 , Aaron M. Whittaker 1
Affiliation
|
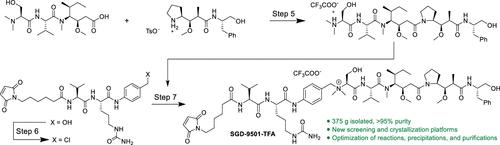
The discovery of novel auristatin-derived antibody drug conjugates (ADCs) with attenuated bystander activity is an area of intense research. Recently, drug-linker SGD-9501-TFA emerged as a promising clinical candidate possessing a favorable off-target toxicity profile. To support the clinical development of ADCs utilizing this drug linker, we set out to develop a first-in-human amenable Good Manufacturing Practice manufacturing route. In this report, we describe the discovery and development of three of seven synthetic steps in convergent solution-phase synthesis. The activation of the linker is achieved with SOCl2 in NMP (step 6), and solutions to the challenges associated with isolation and stability are described. Next, novel HTE platforms used to explore peptide coupling and crystallization for the synthesis of the payload, auristatin S, are unveiled (step 5). Finally, the synthesis of the quaternary ammonium drug linker, SGD-9501-TFA, with a NaI-mediated benzylic amination, is described (step 7). We also discuss solutions to a eutectic gelation risk to direct precipitation of the crude drug linker and a trifluoroacetate ester impurity forming during lyophilization.
中文翻译:

SGD-9501-TFA 首次 GMP 合成的工艺开发,第 2 部分:有效载荷、接头和药物接头的合成
发现具有减弱旁观者活性的新型 auristatin 衍生抗体药物偶联物 (ADC) 是一个深入研究的领域。最近,药物接头 SGD-9501-TFA 成为一种有前途的临床候选药物,具有良好的脱靶毒性特征。为了支持利用这种药物连接子的 ADC 的临床开发,我们着手开发一种首次在人体中适合的良好生产规范生产路线。在本报告中,我们描述了收敛溶液相合成中 7 个合成步骤中 3 个合成步骤的发现和开发。在 NMP 中通过 SOCl2 实现接头的激活(步骤 6),并描述了与分离和稳定性相关的挑战的解决方案。接下来,揭开了用于探索肽偶联和结晶以合成有效载荷 auristatin S 的新型 HTE 平台(步骤 5)。最后,描述了季铵盐药物接头 SGD-9501-TFA 与 NaI 介导的苄基胺化的合成(步骤 7)。我们还讨论了粗药接头直接沉淀的共晶凝胶风险和冻干过程中形成三氟乙酸酯杂质的解决方案。
更新日期:2024-09-13
中文翻译:

SGD-9501-TFA 首次 GMP 合成的工艺开发,第 2 部分:有效载荷、接头和药物接头的合成
发现具有减弱旁观者活性的新型 auristatin 衍生抗体药物偶联物 (ADC) 是一个深入研究的领域。最近,药物接头 SGD-9501-TFA 成为一种有前途的临床候选药物,具有良好的脱靶毒性特征。为了支持利用这种药物连接子的 ADC 的临床开发,我们着手开发一种首次在人体中适合的良好生产规范生产路线。在本报告中,我们描述了收敛溶液相合成中 7 个合成步骤中 3 个合成步骤的发现和开发。在 NMP 中通过 SOCl2 实现接头的激活(步骤 6),并描述了与分离和稳定性相关的挑战的解决方案。接下来,揭开了用于探索肽偶联和结晶以合成有效载荷 auristatin S 的新型 HTE 平台(步骤 5)。最后,描述了季铵盐药物接头 SGD-9501-TFA 与 NaI 介导的苄基胺化的合成(步骤 7)。我们还讨论了粗药接头直接沉淀的共晶凝胶风险和冻干过程中形成三氟乙酸酯杂质的解决方案。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号