当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Axially Chiral Monofluoroalkenes via Nickel-Catalyzed Reductive Cross-Coupling of gem-Difluoroalkenes
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.orglett.4c03119 Tiantian Yin 1 , Ming Jin 1 , Tiantian Zhao 1 , Junbiao Chang 1 , Dachang Bai 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.orglett.4c03119 Tiantian Yin 1 , Ming Jin 1 , Tiantian Zhao 1 , Junbiao Chang 1 , Dachang Bai 1
Affiliation
|
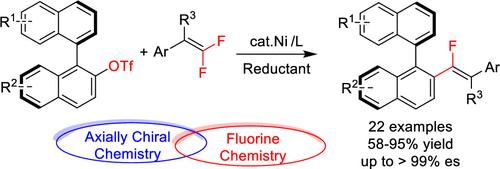
Enantioenriched monofluoroalkenes are important structural motifs in life science and functional materials. To date, only limited strategies were reported for the synthesis of monofluoroalkenes with stereogenic carbon centers; the axially chiral counterpart is still highly desirable. Herein, we report Ni-catalyzed defluorinative cross-electrophile coupling of gem-difluoroalkenes with biaryl electrophiles for the synthesis of axially chiral monofluoroalkenes. The resulting axially chiral monofluoroalkenes are formed with excellent regio- and stereoselectivities. Synthetic transformation of these axially enantioenriched monofluoroalkenes was also demonstrated.
中文翻译:

镍催化偕二氟烯烃还原交叉偶联合成轴向手性单氟烯烃
对映体富集的单氟烯烃是生命科学和功能材料中的重要结构基序。迄今为止,仅报道了有限的具有立体碳中心的单氟烯烃的合成策略;轴向手性对应物仍然是非常理想的。在此,我们报道了镍催化偕二氟烯烃与联芳基亲电试剂的脱氟交叉亲电试剂偶联,用于合成轴向手性单氟烯烃。所得的轴向手性单氟烯烃具有优异的区域选择性和立体选择性。还证明了这些轴向对映体富集的单氟烯烃的合成转化。
更新日期:2024-09-13
中文翻译:

镍催化偕二氟烯烃还原交叉偶联合成轴向手性单氟烯烃
对映体富集的单氟烯烃是生命科学和功能材料中的重要结构基序。迄今为止,仅报道了有限的具有立体碳中心的单氟烯烃的合成策略;轴向手性对应物仍然是非常理想的。在此,我们报道了镍催化偕二氟烯烃与联芳基亲电试剂的脱氟交叉亲电试剂偶联,用于合成轴向手性单氟烯烃。所得的轴向手性单氟烯烃具有优异的区域选择性和立体选择性。还证明了这些轴向对映体富集的单氟烯烃的合成转化。































 京公网安备 11010802027423号
京公网安备 11010802027423号