当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Capture and Traceless Release of Functional CD8+ T Cells with a Microfluidic Chip for Enhanced In Vitro and In Vivo CD4-CAR Transduction
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.analchem.4c03135 Aynur Abdulla 1, 2 , Tuersunayi Abudureheman 3 , Kaiming Chen 3 , Behafarid Ghalandari 2 , Haoni Yan 1 , Hang Zhou 3 , Hengxing Su 4 , Yunqian Zhang 1 , Cai-Wen Duan 3 , Xianting Ding 1, 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.analchem.4c03135 Aynur Abdulla 1, 2 , Tuersunayi Abudureheman 3 , Kaiming Chen 3 , Behafarid Ghalandari 2 , Haoni Yan 1 , Hang Zhou 3 , Hengxing Su 4 , Yunqian Zhang 1 , Cai-Wen Duan 3 , Xianting Ding 1, 2
Affiliation
|
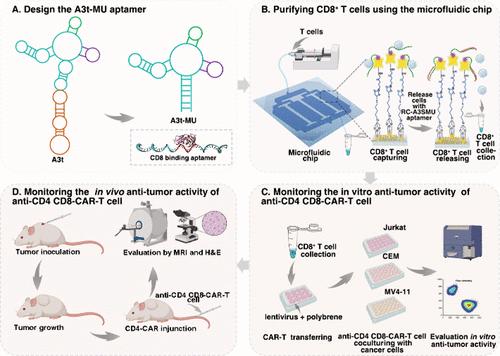
The chimeric antigen receptor (CAR) T cells targeting CD4 expressed cells in acute lymphoblastic leukemia (T-ALL) and acute myeloid leukemia (AML) could reduce the risk of off target effects in normal tissues. However, the efficacy of adoptive cell therapy is predominantly attributed to CD8+ T cells, necessitating their purification before lentivirus transfection to enhance the production of CD4-CAR-T cells. In this study, we developed a microfluidic chip functionalized with an optimized CD8 aptamer, A3t-MU, to facilitate the enrichment and purification of CD8+ T cells. The presented chip showed efficient capture and seamless release of CD8+ T cells from cultured T cells and peripheral blood mononuclear cells (PBMCs). The purity of the released CD8+ T cells reached 98.1%, representing a 13% improvement over the conventional magnetic bead separation method. CD4-CAR was efficiently transduced into the purified CD8+ T cells to construct CAR-T cells. We evaluated the antitumor capability of the CD4-CAR transduced CD8+ T cells (anti-CD4 CD8-CAR T cells) both in vitro and in vivo. The anti-CD4 CD8-CAR T cells exhibited significant cancer-cell-killing capacity across multiple tumor cell lines, including CEM, Jurkat, and MV4-11. Meanwhile, anti-CD4 CD8-CAR T cells significantly inhibited tumor growth in vivo. In conclusion, the presented microfluidic chip offers a cost-effective and high-purity approach for CD8+ T cell separation, enhancing CD4-CAR transduction and achieving efficient antitumor capability both in vitro and in vivo.
中文翻译:

使用微流控芯片有效捕获和无痕释放功能性 CD8+ T 细胞,增强体外和体内 CD4-CAR 转导
针对急性淋巴细胞白血病(T-ALL)和急性髓性白血病(AML)中CD4表达细胞的嵌合抗原受体(CAR)T细胞可以降低正常组织中脱靶效应的风险。然而,过继细胞疗法的功效主要归因于CD8 + T细胞,因此需要在慢病毒转染之前对其进行纯化,以增强CD4-CAR-T细胞的产生。在本研究中,我们开发了一种具有优化的 CD8 适体 A3t-MU 功能的微流控芯片,以促进 CD8 + T 细胞的富集和纯化。该芯片显示出从培养的 T 细胞和外周血单核细胞 (PBMC) 中有效捕获和无缝释放 CD8 + T 细胞。释放的CD8 + T细胞纯度达到98.1%,比传统磁珠分离方法提高了13%。 CD4-CAR被有效转导到纯化的CD8 + T细胞中,构建CAR-T细胞。我们在体外和体内评估了 CD4-CAR 转导的 CD8 + T 细胞(抗 CD4 CD8-CAR T 细胞)的抗肿瘤能力。抗 CD4 CD8-CAR T 细胞在多种肿瘤细胞系(包括 CEM、Jurkat 和 MV4-11)中表现出显着的癌细胞杀伤能力。同时,抗CD4 CD8-CAR T细胞在体内显着抑制肿瘤生长。总之,所提出的微流控芯片为 CD8 + T 细胞分离提供了一种经济高效且高纯度的方法,增强了 CD4-CAR 转导并在体外和体内实现高效的抗肿瘤能力。
更新日期:2024-09-13
中文翻译:

使用微流控芯片有效捕获和无痕释放功能性 CD8+ T 细胞,增强体外和体内 CD4-CAR 转导
针对急性淋巴细胞白血病(T-ALL)和急性髓性白血病(AML)中CD4表达细胞的嵌合抗原受体(CAR)T细胞可以降低正常组织中脱靶效应的风险。然而,过继细胞疗法的功效主要归因于CD8 + T细胞,因此需要在慢病毒转染之前对其进行纯化,以增强CD4-CAR-T细胞的产生。在本研究中,我们开发了一种具有优化的 CD8 适体 A3t-MU 功能的微流控芯片,以促进 CD8 + T 细胞的富集和纯化。该芯片显示出从培养的 T 细胞和外周血单核细胞 (PBMC) 中有效捕获和无缝释放 CD8 + T 细胞。释放的CD8 + T细胞纯度达到98.1%,比传统磁珠分离方法提高了13%。 CD4-CAR被有效转导到纯化的CD8 + T细胞中,构建CAR-T细胞。我们在体外和体内评估了 CD4-CAR 转导的 CD8 + T 细胞(抗 CD4 CD8-CAR T 细胞)的抗肿瘤能力。抗 CD4 CD8-CAR T 细胞在多种肿瘤细胞系(包括 CEM、Jurkat 和 MV4-11)中表现出显着的癌细胞杀伤能力。同时,抗CD4 CD8-CAR T细胞在体内显着抑制肿瘤生长。总之,所提出的微流控芯片为 CD8 + T 细胞分离提供了一种经济高效且高纯度的方法,增强了 CD4-CAR 转导并在体外和体内实现高效的抗肿瘤能力。































 京公网安备 11010802027423号
京公网安备 11010802027423号