当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Synthesis of Sugar Amides by Aerobic One-Pot Oxidative Amidation of Unprotected Carbohydrates
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-09-11 , DOI: 10.1021/acssuschemeng.4c05016 Lucie Quéhon 1 , Abed Bil 1 , Frédéric Sauvage 2 , Anne Wadouachi 1 , Gwladys Pourceau 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-09-11 , DOI: 10.1021/acssuschemeng.4c05016 Lucie Quéhon 1 , Abed Bil 1 , Frédéric Sauvage 2 , Anne Wadouachi 1 , Gwladys Pourceau 1
Affiliation
|
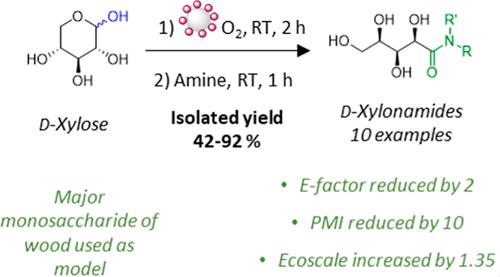
The development of eco-compatible and economically competitive methods enabling carbohydrate transformation into high-added value sugar-based derivatives is highly desirable to promote their valorization as petroleum-based chemical alternatives. We propose in this study a one-pot sequential procedure for the synthesis of sugar amides, used in various areas from detergency to medicine, starting from nonprotected abundant carbohydrates. The use of a small amount of a recyclable gold catalyst (0.36 mol % Au, Au/CeO2) is crucial for promoting the oxidative amidation reaction. In the presence of benzylamine, d-xylose (the main monomer of hemicellulose used as a model) was converted into corresponding benzyl xylonamide in 3 h at room temperature under an O2 atmosphere. The optimized conditions were then successfully applied to a series of abundant carbohydrates (glucose, galactose, and maltose) and a wide range of amines, thus demonstrating the procedure’s versatility. It is established as not sugar-dependent while being compatible with various functional groups such as alkene, alkyne, thiol, and hydroxyl. Finally, the greenness of the procedure was assessed by comparing the synthesis of N-decyl-d-xylonamide using four green metrics, underlining lower waste generation, higher atom economy, and more acceptable reaction conditions in terms of environmental impact.
中文翻译:

无保护碳水化合物的有氧一锅氧化酰胺化直接合成糖酰胺
开发生态兼容且具有经济竞争力的方法,使碳水化合物能够转化为高附加值的糖基衍生物,对于促进其作为石油基化学替代品的价值是非常必要的。我们在这项研究中提出了一种从未受保护的丰富碳水化合物开始合成糖酰胺的一锅连续程序,该程序用于从洗涤剂到医药的各个领域。使用少量可回收的金催化剂(0.36 mol% Au, Au/CeO 2 )对于促进氧化酰胺化反应至关重要。在苄胺存在下, d-木糖(用作模型的半纤维素的主要单体)在室温和O 2气氛下在3小时内转化为相应的苄基木糖酰胺。然后,优化的条件成功应用于一系列丰富的碳水化合物(葡萄糖、半乳糖和麦芽糖)和多种胺,从而证明了该过程的多功能性。它被认为不依赖于糖,同时与各种官能团(例如烯烃、炔烃、硫醇和羟基)相容。最后,通过使用四种绿色指标比较N-癸基-d-木酰胺的合成来评估该过程的绿色性,强调废物产生量更低、原子经济性更高以及在环境影响方面更可接受的反应条件。
更新日期:2024-09-11
中文翻译:

无保护碳水化合物的有氧一锅氧化酰胺化直接合成糖酰胺
开发生态兼容且具有经济竞争力的方法,使碳水化合物能够转化为高附加值的糖基衍生物,对于促进其作为石油基化学替代品的价值是非常必要的。我们在这项研究中提出了一种从未受保护的丰富碳水化合物开始合成糖酰胺的一锅连续程序,该程序用于从洗涤剂到医药的各个领域。使用少量可回收的金催化剂(0.36 mol% Au, Au/CeO 2 )对于促进氧化酰胺化反应至关重要。在苄胺存在下, d-木糖(用作模型的半纤维素的主要单体)在室温和O 2气氛下在3小时内转化为相应的苄基木糖酰胺。然后,优化的条件成功应用于一系列丰富的碳水化合物(葡萄糖、半乳糖和麦芽糖)和多种胺,从而证明了该过程的多功能性。它被认为不依赖于糖,同时与各种官能团(例如烯烃、炔烃、硫醇和羟基)相容。最后,通过使用四种绿色指标比较N-癸基-d-木酰胺的合成来评估该过程的绿色性,强调废物产生量更低、原子经济性更高以及在环境影响方面更可接受的反应条件。

































 京公网安备 11010802027423号
京公网安备 11010802027423号