Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ru Single Atom Dispersed Cu Nanoparticle with Dual Sites Enables Outstanding Photocatalytic CO2 Reduction
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-13 , DOI: 10.1021/acsnano.4c08303 Lizhen Liu 1, 2 , Jingcong Hu 3 , Yuan Sheng 4 , Hossein Akhoundzadeh 2 , Wenguang Tu 5 , Wei Jian Samuel Siow 2, 6 , Jia Hui Ong 2 , Hongwei Huang 1 , Rong Xu 2, 7
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-13 , DOI: 10.1021/acsnano.4c08303 Lizhen Liu 1, 2 , Jingcong Hu 3 , Yuan Sheng 4 , Hossein Akhoundzadeh 2 , Wenguang Tu 5 , Wei Jian Samuel Siow 2, 6 , Jia Hui Ong 2 , Hongwei Huang 1 , Rong Xu 2, 7
Affiliation
|
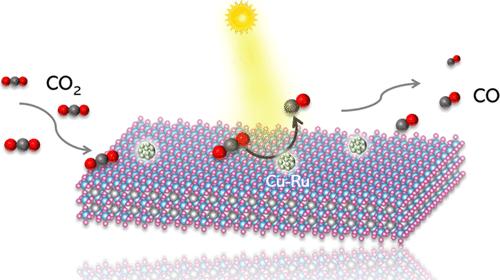
Cu-based catalysts are promising candidates for CO2 reduction owing to the favorable energetics of Cu sites for CO2 adsorption and transformation. However, CO2 reduction involving insurmountable activation barriers and various byproducts remains a significant challenge to achieve high activity and selectivity. Herein, a photocatalyst constructed with single-Ru-site-on-Cu-nanoparticle on Bi4Ti3O12 exhibits exceptional activity and selectivity for CO2 conversion to CO. The experimental and theoretical results consistently reveal that the Ru–Cu dual sites allow the rapid transfer of photogenerated carriers for closely interacting with CO2 molecules. Importantly, the Ru–Cu dual sites exhibit extremely strong CO2 adsorption ability, and the Gibbs free energy of the rate-determining step (*CO2 to *COOH) has been significantly reduced, synergistically enhancing the entire CO2 conversion process. The optimal BTOCu2Ru0.5 photocatalyst manifests a high performance for selective reduction of CO2 to CO, yielding 10.84 μmol over 15 mg of photocatalyst in 4 h (180.67 μmol·g–1·h–1) under a 300 W Xe lamp without any photosensitizer and sacrificial reagent, outperforming all bismuth-based materials and being one of the best photocatalysts ever reported under similar reaction conditions. This work presents a strategy for the rational design of multiple metal sites toward efficient photocatalytic reduction of CO2.
中文翻译:

Ru 单原子分散的双位点铜纳米粒子可实现出色的光催化二氧化碳减排
由于Cu位点对于CO 2吸附和转化有利的能量学,铜基催化剂是CO 2还原的有前途的候选者。然而,涉及难以克服的活化障碍和各种副产物的CO 2还原仍然是实现高活性和选择性的重大挑战。在此,在Bi 4 Ti 3 O 12上用单Ru位点Cu纳米粒子构建的光催化剂对CO 2转化为CO表现出优异的活性和选择性。实验和理论结果一致表明Ru-Cu双位点允许光生载流子的快速转移以与CO 2分子密切相互作用。重要的是,Ru-Cu双位点表现出极强的CO 2吸附能力,并且速率决定步骤(*CO 2到*COOH)的吉布斯自由能显着降低,协同增强了整个CO 2转化过程。最佳的BTOCu 2 Ru 0.5光催化剂表现出将CO 2选择性还原为CO的高性能,在300 W Xe灯下4小时内产生10.84 μmol超过15 mg的光催化剂(180.67 μmol·g –1 ·h –1 )。任何光敏剂和牺牲剂,其性能优于所有铋基材料,是迄今为止在类似反应条件下报道的最好的光催化剂之一。这项工作提出了合理设计多个金属位点以实现高效光催化还原CO 2的策略。
更新日期:2024-09-13
中文翻译:

Ru 单原子分散的双位点铜纳米粒子可实现出色的光催化二氧化碳减排
由于Cu位点对于CO 2吸附和转化有利的能量学,铜基催化剂是CO 2还原的有前途的候选者。然而,涉及难以克服的活化障碍和各种副产物的CO 2还原仍然是实现高活性和选择性的重大挑战。在此,在Bi 4 Ti 3 O 12上用单Ru位点Cu纳米粒子构建的光催化剂对CO 2转化为CO表现出优异的活性和选择性。实验和理论结果一致表明Ru-Cu双位点允许光生载流子的快速转移以与CO 2分子密切相互作用。重要的是,Ru-Cu双位点表现出极强的CO 2吸附能力,并且速率决定步骤(*CO 2到*COOH)的吉布斯自由能显着降低,协同增强了整个CO 2转化过程。最佳的BTOCu 2 Ru 0.5光催化剂表现出将CO 2选择性还原为CO的高性能,在300 W Xe灯下4小时内产生10.84 μmol超过15 mg的光催化剂(180.67 μmol·g –1 ·h –1 )。任何光敏剂和牺牲剂,其性能优于所有铋基材料,是迄今为止在类似反应条件下报道的最好的光催化剂之一。这项工作提出了合理设计多个金属位点以实现高效光催化还原CO 2的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号