Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strong Electronic Metal–Support Interactions Enable the Increased Spin State of Co–N4 Active Sites and Performance for Acidic Oxygen Reduction Reaction
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-12 , DOI: 10.1021/acsnano.4c06615 Miao-Ying Chen , Shuhu Yin 1 , Gen Li 2 , Junxiang Chen 3 , Wen-Yuan Zhao 4 , Yi-Kai Lian 4 , Hao-Ran Wu 4 , Wenfu Yan 5 , Jia-Nan Zhang 4 , Bang-An Lu 4
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-12 , DOI: 10.1021/acsnano.4c06615 Miao-Ying Chen , Shuhu Yin 1 , Gen Li 2 , Junxiang Chen 3 , Wen-Yuan Zhao 4 , Yi-Kai Lian 4 , Hao-Ran Wu 4 , Wenfu Yan 5 , Jia-Nan Zhang 4 , Bang-An Lu 4
Affiliation
|
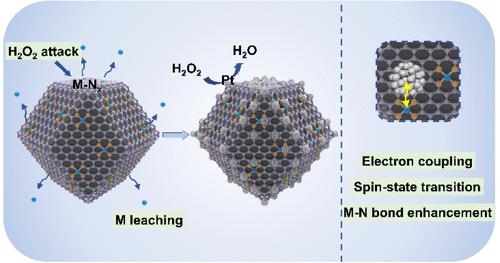
Nonprecious metal catalysts, particularly M–N–C catalysts, are widely recognized as promising contenders for the oxygen reduction reaction (ORR). However, a notable performance gap persists between M–N–C catalysts and Pt-based catalysts under acidic conditions. In this study, hybrid catalysts comprising single Co atoms and ultralow concentrations of Pt3Co intermetallic nanoparticles (NPs) are introduced to enhance ORR performance. Under acidic conditions, these hybrid catalysts demonstrate ORR efficiency with a half-wave potential of 0.895 V, negligible decay even after 80 000 cycles, and a high maximum power density of 1.34 W cm–2 in fuel cells. This performance surpasses those of Co–N–C and Pt/Co–N–C catalysts. Both experimental findings and theoretical computations suggest that the heightened ORR activity stems from an increase in the spin density of Co sites induced by noble metal NPs, facilitating the activation of O–O bonds via side-on overlapping and enabling a transition in the reaction pathway from associative to dissociative processes. This research offers a promising avenue for the systematic design of M–N–C cathodes with an enhanced performance for acidic fuel cells.
中文翻译:

强电子金属-载体相互作用可提高 Co-N4 活性位点的自旋态和酸性氧还原反应的性能
非贵金属催化剂,特别是M -N-C 催化剂,被广泛认为是氧还原反应(ORR)的有希望的竞争者。然而,在酸性条件下, M -N-C 催化剂和 Pt 基催化剂之间仍然存在显着的性能差距。在这项研究中,引入了包含单个 Co 原子和超低浓度 Pt 3 Co 金属间纳米颗粒 (NP) 的混合催化剂,以提高 ORR 性能。在酸性条件下,这些混合催化剂在燃料电池中表现出半波电位为 0.895 V 的 ORR 效率,即使在 80 000 次循环后衰减也可以忽略不计,并且最大功率密度高达 1.34 W cm –2 。该性能超过了 Co-N-C 和 Pt/Co-N-C 催化剂。实验结果和理论计算都表明,ORR活性的提高源于贵金属纳米粒子诱导的Co位点自旋密度的增加,通过侧向重叠促进O-O键的活化,并实现反应途径的转变从联想到解离的过程。这项研究为M -N-C 阴极的系统设计提供了一条有前景的途径,增强了酸性燃料电池的性能。
更新日期:2024-09-12
中文翻译:

强电子金属-载体相互作用可提高 Co-N4 活性位点的自旋态和酸性氧还原反应的性能
非贵金属催化剂,特别是M -N-C 催化剂,被广泛认为是氧还原反应(ORR)的有希望的竞争者。然而,在酸性条件下, M -N-C 催化剂和 Pt 基催化剂之间仍然存在显着的性能差距。在这项研究中,引入了包含单个 Co 原子和超低浓度 Pt 3 Co 金属间纳米颗粒 (NP) 的混合催化剂,以提高 ORR 性能。在酸性条件下,这些混合催化剂在燃料电池中表现出半波电位为 0.895 V 的 ORR 效率,即使在 80 000 次循环后衰减也可以忽略不计,并且最大功率密度高达 1.34 W cm –2 。该性能超过了 Co-N-C 和 Pt/Co-N-C 催化剂。实验结果和理论计算都表明,ORR活性的提高源于贵金属纳米粒子诱导的Co位点自旋密度的增加,通过侧向重叠促进O-O键的活化,并实现反应途径的转变从联想到解离的过程。这项研究为M -N-C 阴极的系统设计提供了一条有前景的途径,增强了酸性燃料电池的性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号