当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Core-Shell Ir@TiO2 Nanoparticles through Hydrogen Treatment as Acidic Oxygen Evolution Reaction Catalysts
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-09-13 , DOI: 10.1002/adfm.202408848 Jihyeon Park 1 , Eric Liu 1 , Shayan Angizi 1 , Ahmed Abdellah 1 , Ecem Yelekli Kirici 1 , Drew Higgins 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-09-13 , DOI: 10.1002/adfm.202408848 Jihyeon Park 1 , Eric Liu 1 , Shayan Angizi 1 , Ahmed Abdellah 1 , Ecem Yelekli Kirici 1 , Drew Higgins 1
Affiliation
|
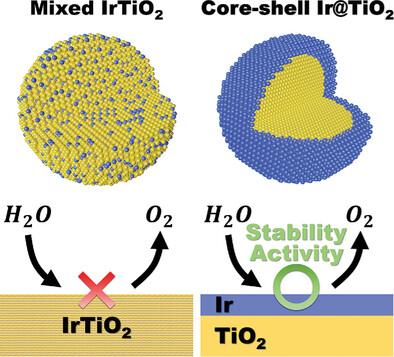
The transition to a sustainable energy economy requires the availability of renewably produced hydrogen through proton exchange membrane water electrolysis. The techno-economic viability of this technology requires addressing materials challenges regarding the lack of active and stable catalysts for the electrochemical oxygen evolution reaction (OER) in acidic conditions. Herein, core-shell iridium/titanium dioxide (Core-shell Ir@TiO2) catalysts for acidic OER are synthesized through a polyol method to create TiO2 nanoparticles, followed by urea reduction with Ir, and subsequent annealing in hydrogen. The formation process of the core-shell structure is observed through in situ environmental transmission electron microscopy under annealing conditions. Ir segregation occurred from an initially blended mixed metal oxide structure to a core-shell configuration at 500 °C. Core-shell Ir@TiO2 showed a three-fold higher stability number (i.e., S-number) than commercial IrOx (3.34 × 106 versus 1.02 × 106). Furthermore, an Ir-mass normalized activity of 1,880 A gIr−1 at 1.7 V versus RHE is measured for Core-shell Ir@TiO2, compared to 624 A gIr−1 for commercial IrOx. The developed synthetic route to prepare a composite structure with a TiO2 core and Ir-based shell has enabled an Ir content reduction without a compromise in activity and stability, thus offering a promising avenue for developing next-generation catalysts tailored for acidic water electrolysis.
中文翻译:

通过氢处理形成核壳Ir@TiO2纳米颗粒作为酸性析氧反应催化剂
向可持续能源经济的过渡需要通过质子交换膜水电解获得可再生生产的氢气。这项技术的技术经济可行性需要解决有关酸性条件下电化学析氧反应 (OER) 缺乏活性和稳定催化剂的材料挑战。本文通过多元醇法合成用于酸性 OER 的核壳铱/二氧化钛 (Core-shell Ir@TiO2) 催化剂,以产生 TiO2 纳米颗粒,然后用 Ir 还原尿素,随后在氢中退火。在退火条件下,通过原位环境透射电子显微镜观察核壳结构的形成过程。在 500 °C 时,Ir 偏析从最初混合的混合金属氧化物结构到核壳构型。 核壳 Ir@TiO2 的稳定性数(即 S 值)比商业 IrOx 高三倍(3.34 × 106 对 1.02 × 106)。此外,核壳 Ir@TiO2 在 1.7 V 下测得的 Ir 质量归一化活度为 1,880 A g Ir-1 与 RHE 相比,而商业 IrOx 的归一化活度为 624 A g Ir-1。开发的合成路线制备了具有 TiO2 核和 Ir 基壳层的复合结构,能够在不影响活性和稳定性的情况下降低 Ir 含量,从而为开发专为酸性水电解量身定制的下一代催化剂提供了一条有前途的途径。
更新日期:2024-09-13
中文翻译:

通过氢处理形成核壳Ir@TiO2纳米颗粒作为酸性析氧反应催化剂
向可持续能源经济的过渡需要通过质子交换膜水电解获得可再生生产的氢气。这项技术的技术经济可行性需要解决有关酸性条件下电化学析氧反应 (OER) 缺乏活性和稳定催化剂的材料挑战。本文通过多元醇法合成用于酸性 OER 的核壳铱/二氧化钛 (Core-shell Ir@TiO2) 催化剂,以产生 TiO2 纳米颗粒,然后用 Ir 还原尿素,随后在氢中退火。在退火条件下,通过原位环境透射电子显微镜观察核壳结构的形成过程。在 500 °C 时,Ir 偏析从最初混合的混合金属氧化物结构到核壳构型。 核壳 Ir@TiO2 的稳定性数(即 S 值)比商业 IrOx 高三倍(3.34 × 106 对 1.02 × 106)。此外,核壳 Ir@TiO2 在 1.7 V 下测得的 Ir 质量归一化活度为 1,880 A g Ir-1 与 RHE 相比,而商业 IrOx 的归一化活度为 624 A g Ir-1。开发的合成路线制备了具有 TiO2 核和 Ir 基壳层的复合结构,能够在不影响活性和稳定性的情况下降低 Ir 含量,从而为开发专为酸性水电解量身定制的下一代催化剂提供了一条有前途的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号