当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Production of Carbonate Anions from Water Electrolysis and CO2 Absorption for Li2CO3 Crystallization from Preconcentrated Brines
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.iecr.4c02193 Nadia C. Zeballos 1, 2 , Walter R. Torres 1 , César H. Díaz Nieto 1 , Victoria Flexer 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.iecr.4c02193 Nadia C. Zeballos 1, 2 , Walter R. Torres 1 , César H. Díaz Nieto 1 , Victoria Flexer 1
Affiliation
|
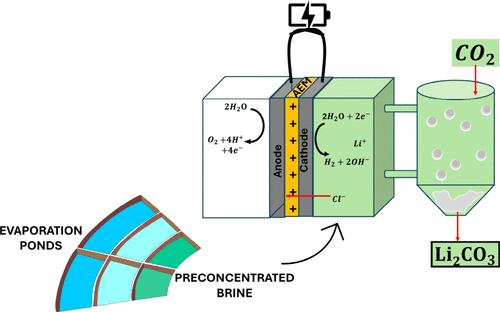
Lithium carbonate (Li2CO3) is normally crystallized from concentrated brines via the addition of sodium carbonate (Na2CO3, soda ash). While this is a methodology largely proved and in implementation in almost all facilities in operation today, it is a chemical intensive procedure. Both the associated costs of sodium carbonate and the logistic issues of bringing large amounts of chemicals to the remote locations where lithium mining from brines takes place should be considered. Here a new prospective technology is proposed for the in situ production of carbonate anions via water reduction and direct CO2 absorption on alkaline media. Experiments were performed on a real concentrated brine sample obtained from an operating facility in the Lithium Triangle. In the first electrolysis, traces of divalent cations were removed with only 1.3% of the total Li+ content in the original brine being trapped in the obtained solid. The second electrolysis produced removal from the brine from 67.2% of the originally contained Li+. Studies were conducted to improve the purification of the solid, since in the highly saline brine (total dissolved solids of 412 g L–1) the as obtained solid was only 70.5% pure. Upon 2 purification steps, a solid 99.1% pure could be obtained. A third electrolysis step could crystallize further 11.8% extra of the original Li+ content, albeit at a much higher energy cost. Upon brine treatment, 88.0 g CO2 per liter of brine fed to the first electrolysis could be absorbed; 63.2 g of which remain in the Li+ depleted brine, and only 24.8 g in the obtained solids.
中文翻译:

通过水电解和 CO2 吸收原位生产碳酸根阴离子,以从预浓缩盐水中结晶 Li2CO3
碳酸锂(Li 2 CO 3 )通常通过添加碳酸钠(Na 2 CO 3 、苏打灰)从浓盐水中结晶出来。虽然这种方法已得到充分验证,并且已在当今运行的几乎所有设施中实施,但它是一种化学品密集型程序。应考虑碳酸钠的相关成本以及将大量化学品运送到从盐水中开采锂的偏远地区的物流问题。这里提出了一种新的前瞻性技术,通过水还原和在碱性介质上直接吸收CO 2来原位生产碳酸根阴离子。实验是在从锂三角运营设施获得的真实浓缩盐水样品上进行的。在第一次电解中,痕量的二价阳离子被去除,仅原始盐水中总Li +含量的1.3%被捕获在所得固体中。第二次电解从盐水中去除了67.2%的最初含有的Li + 。进行了研究以改善固体的纯化,因为在高盐水(总溶解固体为 412 g L –1 )中,所得固体的纯度仅为 70.5%。经过 2 步纯化,可以获得纯度为 99.1% 的固体。第三个电解步骤可以使原始 Li +含量进一步结晶 11.8%,尽管能源成本要高得多。盐水处理后,每升第一次电解的盐水可吸收88.0g CO 2 ;其中63.2克保留在贫Li +盐水中,并且仅24.8克保留在获得的固体中。
更新日期:2024-09-13
中文翻译:

通过水电解和 CO2 吸收原位生产碳酸根阴离子,以从预浓缩盐水中结晶 Li2CO3
碳酸锂(Li 2 CO 3 )通常通过添加碳酸钠(Na 2 CO 3 、苏打灰)从浓盐水中结晶出来。虽然这种方法已得到充分验证,并且已在当今运行的几乎所有设施中实施,但它是一种化学品密集型程序。应考虑碳酸钠的相关成本以及将大量化学品运送到从盐水中开采锂的偏远地区的物流问题。这里提出了一种新的前瞻性技术,通过水还原和在碱性介质上直接吸收CO 2来原位生产碳酸根阴离子。实验是在从锂三角运营设施获得的真实浓缩盐水样品上进行的。在第一次电解中,痕量的二价阳离子被去除,仅原始盐水中总Li +含量的1.3%被捕获在所得固体中。第二次电解从盐水中去除了67.2%的最初含有的Li + 。进行了研究以改善固体的纯化,因为在高盐水(总溶解固体为 412 g L –1 )中,所得固体的纯度仅为 70.5%。经过 2 步纯化,可以获得纯度为 99.1% 的固体。第三个电解步骤可以使原始 Li +含量进一步结晶 11.8%,尽管能源成本要高得多。盐水处理后,每升第一次电解的盐水可吸收88.0g CO 2 ;其中63.2克保留在贫Li +盐水中,并且仅24.8克保留在获得的固体中。































 京公网安备 11010802027423号
京公网安备 11010802027423号