Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of kinetic asymmetry in a multi-cycle reaction network establishes the principles for autonomous compartmentalized molecular ratchets
Chem ( IF 19.1 ) Pub Date : 2024-09-13 , DOI: 10.1016/j.chempr.2024.07.038 Emanuele Penocchio , Ahmad Bachir , Alberto Credi , Raymond Dean Astumian , Giulio Ragazzon
Chem ( IF 19.1 ) Pub Date : 2024-09-13 , DOI: 10.1016/j.chempr.2024.07.038 Emanuele Penocchio , Ahmad Bachir , Alberto Credi , Raymond Dean Astumian , Giulio Ragazzon
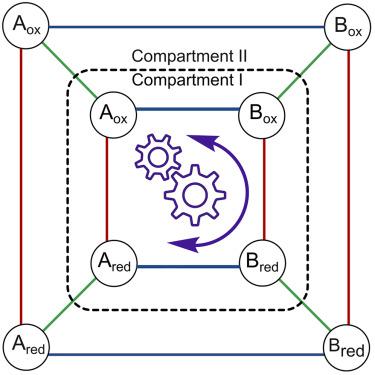
|
Kinetic asymmetry is a key parameter describing non-equilibrium systems: it indicates the directionality of a reaction network under steady-state conditions. So far, kinetic asymmetry has been evaluated only in networks featuring a single cycle. Here, we have investigated kinetic asymmetry in a multi-cycle system using a combined theoretical and numerical approach. First, we report the general expression of kinetic asymmetry for multi-cycle networks. Then, we specify it for a recently reported electrochemically controlled network comprising diffusion steps, which we used as a model system to reveal how key parameters influence directionality. In contrast with the current understanding, we establish that spatial separation—including compartmentalization—can enable autonomous energy ratchet mechanisms, with directionality dictated by thermodynamic features. Kinetic simulations confirm analytical findings and illustrate the interplay between diffusion, chemical, and electrochemical processes. The treatment is general, as it can be applied to other multi-cycle networks, facilitating the realization of endergonic processes across domains.
中文翻译:

多循环反应网络中动力学不对称性的分析确立了自主区室分子棘轮的原理
动力学不对称性是描述非平衡系统的关键参数:它表示稳态条件下反应网络的方向性。到目前为止,仅在具有单个周期的网络中评估了动力学不对称性。在这里,我们使用理论和数值相结合的方法研究了多循环系统中的动力学不对称性。首先,我们报告了多周期网络动力学不对称性的一般表达式。然后,我们为最近报道的包含扩散步骤的电化学控制网络指定它,我们将其用作模型系统来揭示关键参数如何影响方向性。与目前的理解相反,我们确定空间分离(包括区室化)可以实现自主能量棘轮机制,其方向性由热力学特征决定。动力学模拟证实了分析结果,并说明了扩散、化学和电化学过程之间的相互作用。这种处理是通用的,因为它可以应用于其他多周期网络,促进跨领域的内能过程的实现。
更新日期:2024-09-13
中文翻译:

多循环反应网络中动力学不对称性的分析确立了自主区室分子棘轮的原理
动力学不对称性是描述非平衡系统的关键参数:它表示稳态条件下反应网络的方向性。到目前为止,仅在具有单个周期的网络中评估了动力学不对称性。在这里,我们使用理论和数值相结合的方法研究了多循环系统中的动力学不对称性。首先,我们报告了多周期网络动力学不对称性的一般表达式。然后,我们为最近报道的包含扩散步骤的电化学控制网络指定它,我们将其用作模型系统来揭示关键参数如何影响方向性。与目前的理解相反,我们确定空间分离(包括区室化)可以实现自主能量棘轮机制,其方向性由热力学特征决定。动力学模拟证实了分析结果,并说明了扩散、化学和电化学过程之间的相互作用。这种处理是通用的,因为它可以应用于其他多周期网络,促进跨领域的内能过程的实现。































 京公网安备 11010802027423号
京公网安备 11010802027423号