当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Constructing Asymmetric Fe–Nb Diatomic Sites to Enhance ORR Activity and Durability
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-12 , DOI: 10.1021/jacs.4c09642 Rui Sui 1 , Bo Liu 2 , Chang Chen 1 , Xin Tan 1 , Chang He 1 , Dongyue Xin 3 , Bowen Chen 3 , Zhiyuan Xu 1 , Jiazhan Li 4 , Wenxing Chen 5 , Zhongbin Zhuang 3 , Zhenbo Wang 2 , Chen Chen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-12 , DOI: 10.1021/jacs.4c09642 Rui Sui 1 , Bo Liu 2 , Chang Chen 1 , Xin Tan 1 , Chang He 1 , Dongyue Xin 3 , Bowen Chen 3 , Zhiyuan Xu 1 , Jiazhan Li 4 , Wenxing Chen 5 , Zhongbin Zhuang 3 , Zhenbo Wang 2 , Chen Chen 1
Affiliation
|
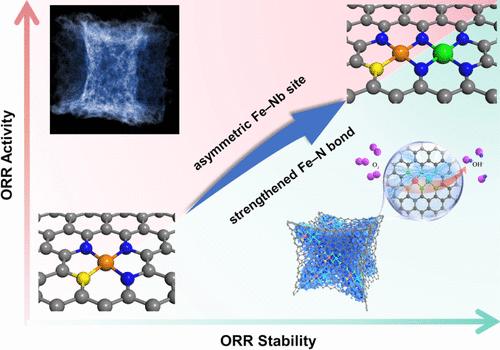
Iron–nitrogen–carbon (Fe–N–C) materials have been identified as a promising class of platinum (Pt)-free catalysts for the oxygen reduction reaction (ORR). However, the dissolution and oxidation of Fe atoms severely restrict their long-term stability and performance. Modulating the active microstructure of Fe–N–C is a feasible strategy to enhance the ORR activity and stability. Compared with common 3d transition metals (Co, Ni, etc.), the 4d transition metal atom Nb has fewer d electrons and more unoccupied orbitals, which could potentially forge a more robust interaction with the Fe site to optimize the binding energy of the oxygen-containing intermediates while maintaining stability. Herein, an asymmetric Fe–Nb diatomic site catalyst (FeNb/c-SNC) was synthesized, which exhibited superior ORR performance and stability compared with those of Fe single-atom catalysts (SACs). The strong interaction within the Fe–Nb diatomic sites optimized the desorption energy of key intermediates (*OH), so that the adsorption energy of Fe–*OH approaches the apex of the volcano plot, thus exhibiting optimal ORR activity. More importantly, introducing Nb atoms could effectively strengthen the Fe–N bonding and suppress Fe demetalation, causing an outstanding stability. The zinc–air battery (ZAB) and hydroxide exchange membrane fuel cell (HEMFC) equipped with our FeNb/c-SNC could deliver high peak power densities of 314 mW cm–2 and 1.18 W cm–2, respectively. Notably, the stable operation time for ZAB and HEMFC increased by 9.1 and 5.8 times compared to Fe SACs, respectively. This research offers further insights into developing stable Fe-based atomic-level catalytic materials for the energy conversion process.
中文翻译:

构建不对称 Fe-Nb 双原子位点以增强 ORR 活性和耐久性
铁-氮-碳(Fe-N-C)材料已被认为是一类有前景的用于氧还原反应(ORR)的无铂(Pt)催化剂。然而,Fe原子的溶解和氧化严重限制了其长期稳定性和性能。调节Fe-N-C的活性微观结构是增强ORR活性和稳定性的可行策略。与常见的3d过渡金属(Co、Ni等)相比,4d过渡金属原子Nb具有更少的d电子和更多的未占据轨道,这可能与Fe位点形成更强大的相互作用,从而优化氧的结合能- 含有中间体,同时保持稳定性。在此,合成了一种不对称Fe-Nb双原子位催化剂(FeNb/c-SNC),与Fe单原子催化剂(SAC)相比,该催化剂表现出优异的ORR性能和稳定性。 Fe-Nb双原子位点内的强相互作用优化了关键中间体(*OH)的解吸能,使得Fe-*OH的吸附能接近火山图的顶点,从而表现出最佳的ORR活性。更重要的是,引入Nb原子可以有效增强Fe-N键合并抑制Fe脱金属,从而具有出色的稳定性。配备我们的FeNb/c-SNC的锌空气电池(ZAB)和氢氧化物交换膜燃料电池(HEMFC)可以分别提供314 mW cm –2和1.18 W cm –2的高峰值功率密度。值得注意的是,与 Fe SAC 相比,ZAB 和 HEMFC 的稳定运行时间分别增加了 9.1 倍和 5.8 倍。这项研究为开发用于能量转换过程的稳定的铁基原子级催化材料提供了进一步的见解。
更新日期:2024-09-12
中文翻译:

构建不对称 Fe-Nb 双原子位点以增强 ORR 活性和耐久性
铁-氮-碳(Fe-N-C)材料已被认为是一类有前景的用于氧还原反应(ORR)的无铂(Pt)催化剂。然而,Fe原子的溶解和氧化严重限制了其长期稳定性和性能。调节Fe-N-C的活性微观结构是增强ORR活性和稳定性的可行策略。与常见的3d过渡金属(Co、Ni等)相比,4d过渡金属原子Nb具有更少的d电子和更多的未占据轨道,这可能与Fe位点形成更强大的相互作用,从而优化氧的结合能- 含有中间体,同时保持稳定性。在此,合成了一种不对称Fe-Nb双原子位催化剂(FeNb/c-SNC),与Fe单原子催化剂(SAC)相比,该催化剂表现出优异的ORR性能和稳定性。 Fe-Nb双原子位点内的强相互作用优化了关键中间体(*OH)的解吸能,使得Fe-*OH的吸附能接近火山图的顶点,从而表现出最佳的ORR活性。更重要的是,引入Nb原子可以有效增强Fe-N键合并抑制Fe脱金属,从而具有出色的稳定性。配备我们的FeNb/c-SNC的锌空气电池(ZAB)和氢氧化物交换膜燃料电池(HEMFC)可以分别提供314 mW cm –2和1.18 W cm –2的高峰值功率密度。值得注意的是,与 Fe SAC 相比,ZAB 和 HEMFC 的稳定运行时间分别增加了 9.1 倍和 5.8 倍。这项研究为开发用于能量转换过程的稳定的铁基原子级催化材料提供了进一步的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号