当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Macrocycles for Enantioselective Recognition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-13 , DOI: 10.1021/jacs.4c07924 Guang Sun 1 , Xue Zhang 1 , Zhe Zheng 1 , Zhi-Yuan Zhang 1 , Ming Dong 1 , Jonathan L Sessler 2 , Chunju Li 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-13 , DOI: 10.1021/jacs.4c07924 Guang Sun 1 , Xue Zhang 1 , Zhe Zheng 1 , Zhi-Yuan Zhang 1 , Ming Dong 1 , Jonathan L Sessler 2 , Chunju Li 1
Affiliation
|
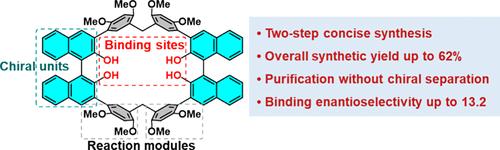
The efficient synthesis of chiral macrocycles with highly enantioselective recognition remains a challenge. We have addressed this issue by synthesizing a pair of chiral macrocycles, namely, R/S-BINOL[2], achieving total isolated yields of up to 62% through a two-step reaction sequence. These macrocycles are readily purified by column chromatography over silica gel without the need for chiral separation, thus streamlining the overall synthesis. R/S-BINOL[2] demonstrated enantioselective recognition toward chiral ammonium salts, with enantioselectivity (KS/KR) values reaching up to 13.2, although less favorable separations were seen for other substrates. R/S-BINOL[2] also displays blue circularly polarized luminescence with a |glum| value of up to 2.2 × 10–3. The R/S-BINOL[2] macrocycles of this study are attractive as chiral hosts in that they both display enantioselective guest recognition and benefit from a concise, high-yielding synthesis. As such, they may have a role to play in chiral separations.
中文翻译:

用于对映选择性识别的手性大环化合物
具有高度对映选择性识别的手性大环化合物的有效合成仍然是一个挑战。我们通过合成一对手性大环化合物,即R / S -BINOL[2] 解决了这个问题,通过两步反应序列实现了高达 62% 的总分离产率。这些大环化合物可以通过硅胶柱色谱法轻松纯化,无需手性分离,从而简化了整个合成过程。 R / S -BINOL[2] 表现出对手性铵盐的对映选择性识别,对映选择性 ( K S / K R ) 值高达 13.2,尽管其他底物的分离效果较差。 R / S -BINOL[2] 还显示蓝色圆偏振发光,具有 |克伦|值高达 2.2 × 10 –3 。本研究的R / S -BINOL[2] 大环化合物作为手性主体很有吸引力,因为它们既表现出对映选择性的客体识别,又受益于简洁、高产的合成。因此,它们可能在手性分离中发挥作用。
更新日期:2024-09-13
中文翻译:

用于对映选择性识别的手性大环化合物
具有高度对映选择性识别的手性大环化合物的有效合成仍然是一个挑战。我们通过合成一对手性大环化合物,即R / S -BINOL[2] 解决了这个问题,通过两步反应序列实现了高达 62% 的总分离产率。这些大环化合物可以通过硅胶柱色谱法轻松纯化,无需手性分离,从而简化了整个合成过程。 R / S -BINOL[2] 表现出对手性铵盐的对映选择性识别,对映选择性 ( K S / K R ) 值高达 13.2,尽管其他底物的分离效果较差。 R / S -BINOL[2] 还显示蓝色圆偏振发光,具有 |克伦|值高达 2.2 × 10 –3 。本研究的R / S -BINOL[2] 大环化合物作为手性主体很有吸引力,因为它们既表现出对映选择性的客体识别,又受益于简洁、高产的合成。因此,它们可能在手性分离中发挥作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号