当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
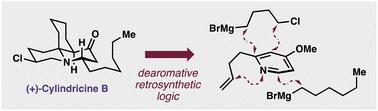
Enantioselective total synthesis of (+)-cylindricine B
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-13 , DOI: 10.1039/d4sc04910a Dallas M. Dukes, Victor K. Atanassov, Joel M. Smith
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-13 , DOI: 10.1039/d4sc04910a Dallas M. Dukes, Victor K. Atanassov, Joel M. Smith
|
This article describes the first enantioselective synthesis of the Tasmanian marine alkaloid (+)-cylindricine B. The concise construction of the compound hinged on dearomative retrosynthetic logic combined with a tactical advance in the generation of congested, cyclic, alpha-tertiary amine centers. The scope of this key coupling reaction was explored in addition to providing a synthetic application for Cu-catalyzed enantioselective dearomatization of N-acyl-pyridiniums. The synthesis proceeds in five or six steps from commercially available starting materials.
中文翻译:

(+)-cylindricine B 的对映选择性全合成
本文描述了塔斯马尼亚海洋生物碱 (+)-cylindricine B 的首次对映选择性合成。该化合物的简洁结构取决于脱芳香逆合成逻辑,并结合生成拥挤、环状 α-叔胺中心的策略进展。除了提供铜催化N-酰基吡啶鎓对映选择性脱芳构化的合成应用之外,还探索了这一关键偶联反应的范围。合成过程采用市售起始原料,分五步或六步进行。
更新日期:2024-09-13
中文翻译:

(+)-cylindricine B 的对映选择性全合成
本文描述了塔斯马尼亚海洋生物碱 (+)-cylindricine B 的首次对映选择性合成。该化合物的简洁结构取决于脱芳香逆合成逻辑,并结合生成拥挤、环状 α-叔胺中心的策略进展。除了提供铜催化N-酰基吡啶鎓对映选择性脱芳构化的合成应用之外,还探索了这一关键偶联反应的范围。合成过程采用市售起始原料,分五步或六步进行。































 京公网安备 11010802027423号
京公网安备 11010802027423号