当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In-Depth Host Cell Protein Analysis and Viral Protein Impurity Monitoring in Adeno-Associated Virus-Based Gene Therapy Products Using Optimized Wide Window Data-Dependent Acquisition Method
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.analchem.4c02400 Shihan Huo 1 , Song Nie 1 , Yongzheng Cong 1 , Shunhai Wang 1 , Ning Li 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.analchem.4c02400 Shihan Huo 1 , Song Nie 1 , Yongzheng Cong 1 , Shunhai Wang 1 , Ning Li 1
Affiliation
|
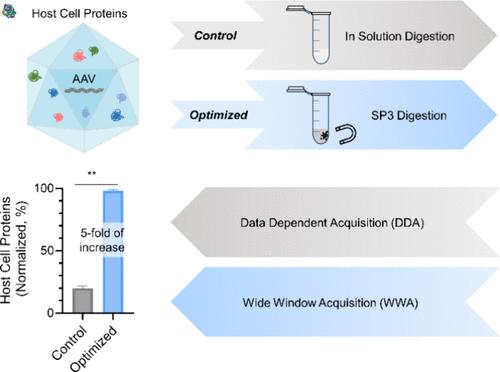
Compared to other protein therapeutics, there is currently limited knowledge about the residual host cell proteins (HCPs) in adeno-associated virus (AAV)-based gene therapy products. This is primarily due to the lack of a robust and sensitive mass spectrometry-based method for HCP analysis in AAV samples. Existing liquid chromatography–mass spectrometry methods used for analyzing HCPs in therapeutic monoclonal antibodies (mAbs) often cannot be directly applied to AAVs, due to some unique characteristics of AAV samples encountered during their development such as limited sample availability/protein concentration and the presence of surfactants. In this study, we have developed a novel workflow for robust and in-depth HCP analysis of AAV samples by combining wide-window data-dependent acquisition for improved low-abundance HCP detection with single-pot, solid-phase-enhanced sample preparation (SP3) for low-input sample preparation. Using this newly developed method, we were able to detect more than 650 HCPs in a commercial AAV1 sample with a high quantitative reproducibility. This represents a greater than 5-fold increase in HCP protein identification compared to an in-solution digestion method followed by traditional data-dependent acquisition. Similar benefits can also be achieved for other AAV serotypes that were produced internally and purified through different processes. The detection limit of this method is as low as 0.06 ng/mL, enabling more comprehensive HCP coverage in AAV samples. Moreover, for the first time, we have identified several process-related viral proteins, such as Rep 78 and E4. These proteins need to be closely monitored during AAV process development as they may present a greater risk for immunogenicity compared to HCPs that are derived from human HEK293 cells.
中文翻译:

使用优化的宽窗口数据依赖性采集方法,对基于腺相关病毒的基因治疗产品进行深入的宿主细胞蛋白质分析和病毒蛋白质杂质监测
与其他蛋白质疗法相比,目前对基于腺相关病毒(AAV)的基因治疗产品中残留宿主细胞蛋白(HCP)的了解有限。这主要是由于缺乏稳健且灵敏的基于质谱的方法来分析 AAV 样品中的 HCP。现有的用于分析治疗性单克隆抗体 (mAb) 中 HCP 的液相色谱-质谱方法通常不能直接应用于 AAV,因为 AAV 样品在开发过程中遇到一些独特的特征,例如有限的样品可用性/蛋白质浓度以及存在表面活性剂。在这项研究中,我们开发了一种新颖的工作流程,通过将宽窗口数据依赖性采集与单锅固相增强样品制备相结合,以改进低丰度 HCP 检测,对 AAV 样品进行稳健和深入的 HCP 分析( SP3) 用于低投入样品制备。使用这种新开发的方法,我们能够以高定量重现性检测商业 AAV1 样本中的 650 多种 HCP。与溶液内消化方法和传统的数据依赖型采集相比,这表明 HCP 蛋白鉴定增加了 5 倍以上。其他内部生产并通过不同工艺纯化的 AAV 血清型也可以获得类似的好处。该方法的检测限低至0.06 ng/mL,能够更全面地覆盖AAV样品中的HCP。此外,我们首次鉴定了几种与过程相关的病毒蛋白,例如Rep 78和E4。 在 AAV 工艺开发过程中需要密切监测这些蛋白质,因为与源自人 HEK293 细胞的 HCP 相比,它们可能会带来更大的免疫原性风险。
更新日期:2024-09-12
中文翻译:

使用优化的宽窗口数据依赖性采集方法,对基于腺相关病毒的基因治疗产品进行深入的宿主细胞蛋白质分析和病毒蛋白质杂质监测
与其他蛋白质疗法相比,目前对基于腺相关病毒(AAV)的基因治疗产品中残留宿主细胞蛋白(HCP)的了解有限。这主要是由于缺乏稳健且灵敏的基于质谱的方法来分析 AAV 样品中的 HCP。现有的用于分析治疗性单克隆抗体 (mAb) 中 HCP 的液相色谱-质谱方法通常不能直接应用于 AAV,因为 AAV 样品在开发过程中遇到一些独特的特征,例如有限的样品可用性/蛋白质浓度以及存在表面活性剂。在这项研究中,我们开发了一种新颖的工作流程,通过将宽窗口数据依赖性采集与单锅固相增强样品制备相结合,以改进低丰度 HCP 检测,对 AAV 样品进行稳健和深入的 HCP 分析( SP3) 用于低投入样品制备。使用这种新开发的方法,我们能够以高定量重现性检测商业 AAV1 样本中的 650 多种 HCP。与溶液内消化方法和传统的数据依赖型采集相比,这表明 HCP 蛋白鉴定增加了 5 倍以上。其他内部生产并通过不同工艺纯化的 AAV 血清型也可以获得类似的好处。该方法的检测限低至0.06 ng/mL,能够更全面地覆盖AAV样品中的HCP。此外,我们首次鉴定了几种与过程相关的病毒蛋白,例如Rep 78和E4。 在 AAV 工艺开发过程中需要密切监测这些蛋白质,因为与源自人 HEK293 细胞的 HCP 相比,它们可能会带来更大的免疫原性风险。

































 京公网安备 11010802027423号
京公网安备 11010802027423号