当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allosteric site identification, virtual screening and discovery of a sulfonamide Hsp110-STAT3 interaction inhibitor for the treatment of hypoxic pulmonary arterial hypertension
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-09-07 , DOI: 10.1016/j.ejmech.2024.116855 Congke Zhao 1 , Yan Wu 1 , Mengqi Li 1 , Wenhua Tan 1 , Yuanbo Hu 1 , Yu Wang 1 , Ruizhe Gao 2 , Liqing Hu 2 , Qianbin Li 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-09-07 , DOI: 10.1016/j.ejmech.2024.116855 Congke Zhao 1 , Yan Wu 1 , Mengqi Li 1 , Wenhua Tan 1 , Yuanbo Hu 1 , Yu Wang 1 , Ruizhe Gao 2 , Liqing Hu 2 , Qianbin Li 1
Affiliation
|
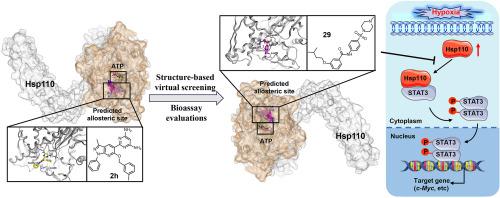
Pulmonary arterial hypertension (PAH) is a severe pulmonary vascular disorder marked by vascular remodeling, which is linked to the malignant phenotypes of pulmonary vascular cells. The prevailing therapeutic approaches for PAH tend to neglect the potential role of vascular remodeling, leading to the clinical prognosis remains poor. Previously, we first demonstrated that heat shock protein (Hsp110) was significantly activated to boost Hsp110-STAT3 interaction, which resulted in abnormal proliferation and migration of human pulmonary arterial endothelial cells (HPAECs) under hypoxia. In the present study, we initially postulated the allosteric site of Hsp110, performed a virtual screening and biological evaluation studies to discover novel Hsp110-STAT3 interaction inhibitors. Here, we identified compound 29 (AN-329/43448068) as the effective inhibitor of HPAECs proliferation and the Hsp110-STAT3 association with good druggability. In vitro , 29 significantly impeded the chaperone function of Hsp110 and the malignant phenotypes of HPAECs. In vivo , 29 remarkably attenuated pulmonary vascular remodeling and right ventricular hypertrophy in hypoxia-induced PAH rats (i.g). Altogether, our data support the conclusion that it not only provides a novel lead compound but also presents a promising approach for subsequent inhibitor development targeting Hsp110-STAT3 interaction.
中文翻译:

用于治疗缺氧性肺动脉高压的磺胺类 Hsp110-STAT3 相互作用抑制剂的变构部位鉴定、虚拟筛选和发现
肺动脉高压 (PAH) 是一种严重的肺血管疾病,其特征是血管重塑,这与肺血管细胞的恶性表型有关。PAH 的主流治疗方法往往忽视了血管重塑的潜在作用,导致临床预后仍然很差。以前,我们首先证明热休克蛋白 (Hsp110) 被显着激活以增强 Hsp110-STAT3 相互作用,这导致人肺动脉内皮细胞 (HPAECs) 在缺氧下异常增殖和迁移。在本研究中,我们最初假设了 Hsp110 的变构位点,进行了虚拟筛选和生物学评价研究,以发现新的 Hsp110-STAT3 相互作用抑制剂。在这里,我们确定化合物 29 (AN-329/43448068) 是 HPAECs 增殖的有效抑制剂,并且 Hsp110-STAT3 具有良好的成药性。在体外,29 个显著阻碍了 Hsp110 的伴侣功能和 HPAECs 的恶性表型。在体内,29 例显著减轻了缺氧诱导的 PAH 大鼠 (即) 的肺血管重塑和右心室肥大。总而言之,我们的数据支持这样一个结论,即它不仅提供了一种新型的先导化合物,而且还为后续靶向 Hsp110-STAT3 相互作用的抑制剂开发提供了一种有前途的方法。
更新日期:2024-09-07
中文翻译:

用于治疗缺氧性肺动脉高压的磺胺类 Hsp110-STAT3 相互作用抑制剂的变构部位鉴定、虚拟筛选和发现
肺动脉高压 (PAH) 是一种严重的肺血管疾病,其特征是血管重塑,这与肺血管细胞的恶性表型有关。PAH 的主流治疗方法往往忽视了血管重塑的潜在作用,导致临床预后仍然很差。以前,我们首先证明热休克蛋白 (Hsp110) 被显着激活以增强 Hsp110-STAT3 相互作用,这导致人肺动脉内皮细胞 (HPAECs) 在缺氧下异常增殖和迁移。在本研究中,我们最初假设了 Hsp110 的变构位点,进行了虚拟筛选和生物学评价研究,以发现新的 Hsp110-STAT3 相互作用抑制剂。在这里,我们确定化合物 29 (AN-329/43448068) 是 HPAECs 增殖的有效抑制剂,并且 Hsp110-STAT3 具有良好的成药性。在体外,29 个显著阻碍了 Hsp110 的伴侣功能和 HPAECs 的恶性表型。在体内,29 例显著减轻了缺氧诱导的 PAH 大鼠 (即) 的肺血管重塑和右心室肥大。总而言之,我们的数据支持这样一个结论,即它不仅提供了一种新型的先导化合物,而且还为后续靶向 Hsp110-STAT3 相互作用的抑制剂开发提供了一种有前途的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号