当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The G Protein-First Mechanism for Activation of the Class B Glucagon-like Peptide 1 Receptor Coupled to N-Terminal Domain-Mediated Conformational Progression
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-12 , DOI: 10.1021/jacs.4c08128 Bo Li 1 , Moon Young Yang 1 , Soo-Kyung Kim 1 , William A Goddard 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-12 , DOI: 10.1021/jacs.4c08128 Bo Li 1 , Moon Young Yang 1 , Soo-Kyung Kim 1 , William A Goddard 1
Affiliation
|
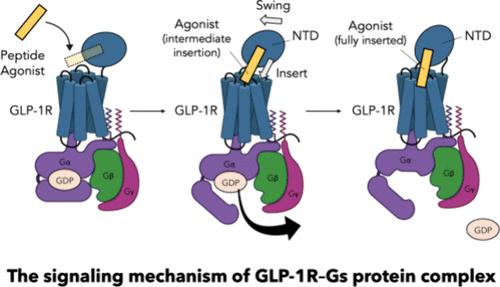
Recently, there has been a great deal of excitement about new glucagon-like peptide 1 receptor (GLP-1R) agonists (e.g., semaglutide and tirzepatide) that have received FDA approval for type 2 diabetes and obesity. Although effective, these drugs come with side effects that limit their use. While research efforts continue to focus intensively on long-lasting, orally administered GLP-1R medications with fewer side effects, a major impediment to developing improved GLP-1R medications is that the mechanism by which an agonist activates GLP-1R to imitate signaling is not known. Here we present and validate the G protein (GP)-first mechanism for the GLP-1R supported by extensive atomistic simulations. We propose that GLP-1R is preactivated through the formation of a GLP-1R–GP precoupled complex at the cell membrane prior to ligand binding. Despite a transmembrane helix 6 (TM6)-bentout conformation characteristic of activated GLP-1R, this precoupled complex remains unactivated until an agonist binds to elicit signaling. Notably, this new hypothesis offers a unified and predictive model for the activities of a series of full and partial agonists, including the peptides ExP5, GLP-1(7-36), and GLP-1(9-36). Most surprisingly, our simulations reveal an N-terminus domain (NTD)-swing/agonist-insertion mechanism wherein the long extracellular NTD of GLP-1R tightly holds the C-terminal half of the peptide agonist and progressively shifts the N-terminal head of the peptide to facilitate insertion into the orthosteric pocket. Our findings provide novel mechanistic insights into the activation and function of class B GPCRs and should provide a realistic basis for structure-based ligand design.
中文翻译:

与 N 端结构域介导的构象进展偶联的 B 类胰高血糖素样肽 1 受体激活的 G 蛋白优先机制
最近,新型胰高血糖素样肽 1 受体 (GLP-1R) 激动剂(例如索马鲁肽和替泽帕肽)已获得 FDA 批准用于治疗 2 型糖尿病和肥胖症,这引起了人们的广泛关注。尽管有效,但这些药物具有限制其使用的副作用。虽然研究工作继续集中于副作用较少的长效口服 GLP-1R 药物,但开发改进的 GLP-1R 药物的一个主要障碍是激动剂激活 GLP-1R 模仿信号传导的机制并不明确。已知。在这里,我们提出并验证了 GLP-1R 的 G 蛋白 (GP) 优先机制,并得到广泛的原子模拟的支持。我们提出,GLP-1R 通过在配体结合之前在细胞膜上形成 GLP-1R-GP 预偶联复合物来预激活。尽管激活的 GLP-1R 具有跨膜螺旋 6 (TM6) 弯曲构象特征,但这种预偶联复合物在激动剂结合引发信号传导之前保持未激活状态。值得注意的是,这一新假设为一系列完全和部分激动剂的活性提供了统一的预测模型,包括肽 ExP5、GLP-1(7-36) 和 GLP-1(9-36)。最令人惊讶的是,我们的模拟揭示了一种 N 末端结构域 (NTD) 摆动/激动剂插入机制,其中 GLP-1R 的长胞外 NTD 紧紧抓住肽激动剂的 C 末端一半,并逐渐移动 GLP-1R 的 N 末端头部。肽以促进插入正位袋。我们的研究结果为 B 类 GPCR 的激活和功能提供了新颖的机制见解,并为基于结构的配体设计提供了现实的基础。
更新日期:2024-09-12
中文翻译:

与 N 端结构域介导的构象进展偶联的 B 类胰高血糖素样肽 1 受体激活的 G 蛋白优先机制
最近,新型胰高血糖素样肽 1 受体 (GLP-1R) 激动剂(例如索马鲁肽和替泽帕肽)已获得 FDA 批准用于治疗 2 型糖尿病和肥胖症,这引起了人们的广泛关注。尽管有效,但这些药物具有限制其使用的副作用。虽然研究工作继续集中于副作用较少的长效口服 GLP-1R 药物,但开发改进的 GLP-1R 药物的一个主要障碍是激动剂激活 GLP-1R 模仿信号传导的机制并不明确。已知。在这里,我们提出并验证了 GLP-1R 的 G 蛋白 (GP) 优先机制,并得到广泛的原子模拟的支持。我们提出,GLP-1R 通过在配体结合之前在细胞膜上形成 GLP-1R-GP 预偶联复合物来预激活。尽管激活的 GLP-1R 具有跨膜螺旋 6 (TM6) 弯曲构象特征,但这种预偶联复合物在激动剂结合引发信号传导之前保持未激活状态。值得注意的是,这一新假设为一系列完全和部分激动剂的活性提供了统一的预测模型,包括肽 ExP5、GLP-1(7-36) 和 GLP-1(9-36)。最令人惊讶的是,我们的模拟揭示了一种 N 末端结构域 (NTD) 摆动/激动剂插入机制,其中 GLP-1R 的长胞外 NTD 紧紧抓住肽激动剂的 C 末端一半,并逐渐移动 GLP-1R 的 N 末端头部。肽以促进插入正位袋。我们的研究结果为 B 类 GPCR 的激活和功能提供了新颖的机制见解,并为基于结构的配体设计提供了现实的基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号