当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of the Phenylnaphthacenoid Type II Polyketide Antibiotic Formicamycin H via Regioselective Ruthenium-Catalyzed Hydrogen Auto-Transfer [4 + 2] Cycloaddition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-12 , DOI: 10.1021/jacs.4c09068 Guanyu Hu 1 , Rosalie S Doerksen 1 , Brett R Ambler 1 , Michael J Krische 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-12 , DOI: 10.1021/jacs.4c09068 Guanyu Hu 1 , Rosalie S Doerksen 1 , Brett R Ambler 1 , Michael J Krische 1
Affiliation
|
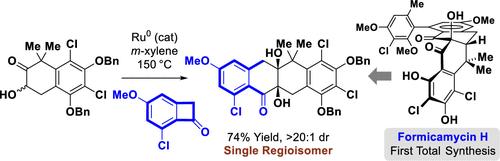
The first total synthesis of the pentacyclic phenylnaphthacenoid type II polyketide antibiotic formicamycin H is described. A key feature of the synthesis involves the convergent, regioselective assembly of the tetracyclic core via ruthenium-catalyzed α-ketol-benzocyclobutenone [4 + 2] cycloaddition. Double dehydration of the diol-containing cycloadduct provides an achiral enone, which upon asymmetric nucleophilic epoxidation and further manipulations delivers the penultimate tetracyclic trichloride in enantiomerically enriched form. Subsequent chemo- and atroposelective Suzuki cross-coupling of the tetracyclic trichloride introduces the E-ring to complete the total synthesis. Single-crystal X-ray diffraction analyses of two model compounds suggest that the initially assigned stereochemistry of the axially chiral C6–C7 linkage may require revision.
中文翻译:

通过区域选择性钌催化的氢自动转移 [4 + 2] 环加成反应完全合成苯基萘素类 II 型聚酮类抗生素福米霉素 H
描述了五环苯基萘藻类 II 型聚酮类抗生素福米霉素 H 的首次全合成。该合成的一个关键特征是通过钌催化的 α-酮-苯并环丁烯酮 [4 + 2] 环加成反应对四环核心进行收敛、区域选择性组装。含二醇的环加合物的双重脱水产生非手性烯酮,在不对称亲核环氧化和进一步操作后,以对映体富集形式提供倒数第二个四环三氯化物。随后四环三氯化物的化学和萎缩选择性 Suzuki 交叉偶联引入 E 环以完成总合成。两种模型化合物的单晶 X 射线衍射分析表明,轴向手性 C6-C7 键的最初分配的立体化学可能需要修订。
更新日期:2024-09-12
中文翻译:

通过区域选择性钌催化的氢自动转移 [4 + 2] 环加成反应完全合成苯基萘素类 II 型聚酮类抗生素福米霉素 H
描述了五环苯基萘藻类 II 型聚酮类抗生素福米霉素 H 的首次全合成。该合成的一个关键特征是通过钌催化的 α-酮-苯并环丁烯酮 [4 + 2] 环加成反应对四环核心进行收敛、区域选择性组装。含二醇的环加合物的双重脱水产生非手性烯酮,在不对称亲核环氧化和进一步操作后,以对映体富集形式提供倒数第二个四环三氯化物。随后四环三氯化物的化学和萎缩选择性 Suzuki 交叉偶联引入 E 环以完成总合成。两种模型化合物的单晶 X 射线衍射分析表明,轴向手性 C6-C7 键的最初分配的立体化学可能需要修订。































 京公网安备 11010802027423号
京公网安备 11010802027423号