当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A General Enantioselective α-Alkyl Amino Acid Derivatives Synthesis Enabled by Cobalt-Catalyzed Reductive Addition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-12 , DOI: 10.1021/jacs.4c09556 Chengxi Zhang 1 , Xianqing Wu 1 , Jingping Qu 1 , Yifeng Chen 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-12 , DOI: 10.1021/jacs.4c09556 Chengxi Zhang 1 , Xianqing Wu 1 , Jingping Qu 1 , Yifeng Chen 1, 2, 3
Affiliation
|
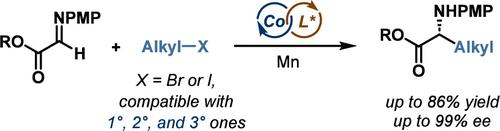
Enantioenriched unnatural amino acids represent a prevalent motif in organic chemistry, with profound applications in biochemistry, medicinal chemistry, and materials science. Herein, we report a cobalt-catalyzed aza-Barbier reaction of dehydroglycines with unactivated alkyl halides to afford unnatural α-amino esters with high enantioselectivity. This catalytic reductive alkylative addition protocol circumvents the use of moisture-, air-sensitive organometallic reagents, and stoichiometric chiral auxiliaries, enabling the conversion of a variety of primary, secondary, and even tertiary unactivated alkyl halides to α-alkyl-amino esters under mild conditions, thus leading to broad functional group tolerance. The expedient access to biologically active motifs demonstrates the practicality of this protocol by reducing the number of synthetic steps and enhancing the reaction efficiency.
中文翻译:

钴催化还原加成合成通用对映选择性 α-烷基氨基酸衍生物
对映体富集的非天然氨基酸代表了有机化学中的普遍主题,在生物化学、药物化学和材料科学中具有深远的应用。在此,我们报道了脱氢甘氨酸与未活化的烷基卤化物的钴催化氮杂巴比尔反应,以提供具有高对映选择性的非天然α-氨基酯。这种催化还原烷基化加成方案避免了使用湿气、空气敏感的有机金属试剂和化学计量的手性助剂,能够在温和的条件下将各种伯、仲、甚至叔未活化的烷基卤化物转化为α-烷基氨基酯。条件,从而导致广泛的官能团耐受性。通过减少合成步骤数和提高反应效率,方便地获取生物活性基序证明了该方案的实用性。
更新日期:2024-09-12
中文翻译:

钴催化还原加成合成通用对映选择性 α-烷基氨基酸衍生物
对映体富集的非天然氨基酸代表了有机化学中的普遍主题,在生物化学、药物化学和材料科学中具有深远的应用。在此,我们报道了脱氢甘氨酸与未活化的烷基卤化物的钴催化氮杂巴比尔反应,以提供具有高对映选择性的非天然α-氨基酯。这种催化还原烷基化加成方案避免了使用湿气、空气敏感的有机金属试剂和化学计量的手性助剂,能够在温和的条件下将各种伯、仲、甚至叔未活化的烷基卤化物转化为α-烷基氨基酯。条件,从而导致广泛的官能团耐受性。通过减少合成步骤数和提高反应效率,方便地获取生物活性基序证明了该方案的实用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号