当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantum Chemical Characterization of Rotamerism in Thio-Michael Additions for Targeted Covalent Inhibitors
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.jcim.4c01379 Shayantan Chaudhuri 1 , David M Rogers 1 , Christopher J Hayes 1 , Katherine Inzani 1 , Jonathan D Hirst 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.jcim.4c01379 Shayantan Chaudhuri 1 , David M Rogers 1 , Christopher J Hayes 1 , Katherine Inzani 1 , Jonathan D Hirst 1
Affiliation
|
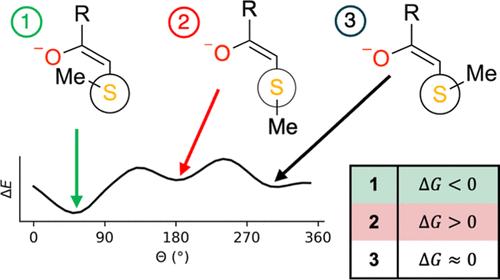
Myotonic dystrophy type I (DM1) is the most common form of adult muscular dystrophy and is a severe condition with no treatment currently available. Recently, small-molecule ligands have been developed as targeted covalent inhibitors that have some selectivity for and covalently inhibit cyclin-dependent kinase 12 (CDK12). CDK12 is involved in the transcription of elongated RNA sections that results in the DM1 condition. The covalent bond is achieved after nucleophilic addition to a Michael acceptor warhead. Previous studies of the conformational preferences of thio-Michael additions have focused on characterizing the reaction profile based on the distance between the sulfur and β-carbon atoms. Rotamerism, however, has not been investigated extensively. Here, we use high-level quantum chemistry calculations, up to coupled cluster with single, double, and perturbative triple excitations [CCSD(T)], to characterize the nucleophilic addition of an archetypal nucleophile, methanethiolate, to various nitrogen-containing Michael acceptors which are representative of the small-molecule covalent inhibitors. By investigating the structural, energetic, and electronic properties of the resulting enolates, as well as their reaction profiles, we show that synclinal additions are generally energetically favored over other additions due to the greater magnitude of attractive noncovalent interactions permitted by the conformation. The calculated transition states associated with the addition process indicate that synclinal addition proceeds via lower energetic barriers than antiperiplanar addition and is the preferred reaction pathway.
中文翻译:

靶向共价抑制剂的 Thio-Michael 添加物中旋转异构的量子化学表征
I 型强直性肌营养不良 (DM1) 是成人肌营养不良症最常见的形式,是一种严重的疾病,目前尚无治疗方法。最近,小分子配体已被开发为靶向共价抑制剂,对细胞周期蛋白依赖性激酶 12 (CDK12) 具有一定的选择性并共价抑制。CDK12 参与导致 DM1 状况的细长 RNA 片段的转录。共价键是在 Michael 受体弹头中亲核添加后实现的。以前对硫-迈克尔加成物构象偏好的研究集中在根据硫原子和 β-碳原子之间的距离来表征反应曲线。然而,Rotamerism 尚未得到广泛研究。在这里,我们使用高级量子化学计算,直到具有单、双和扰动三重激发 [CCSD(T)] 的耦合簇,以表征原型亲核试剂甲烷硫代酸盐对各种含氮 Michael 受体的亲核添加,这些受体是代表小分子共价抑制剂。通过研究所得烯醇化物的结构、能量和电子性质以及它们的反应曲线,我们表明,由于构象允许的有吸引力的非共价相互作用的幅度更大,因此在能量上通常比其他加成更有利。与加成过程相关的计算过渡态表明,与反平面加成相比,同步加成通过更低的能量屏障进行,并且是首选的反应途径。
更新日期:2024-09-12
中文翻译:

靶向共价抑制剂的 Thio-Michael 添加物中旋转异构的量子化学表征
I 型强直性肌营养不良 (DM1) 是成人肌营养不良症最常见的形式,是一种严重的疾病,目前尚无治疗方法。最近,小分子配体已被开发为靶向共价抑制剂,对细胞周期蛋白依赖性激酶 12 (CDK12) 具有一定的选择性并共价抑制。CDK12 参与导致 DM1 状况的细长 RNA 片段的转录。共价键是在 Michael 受体弹头中亲核添加后实现的。以前对硫-迈克尔加成物构象偏好的研究集中在根据硫原子和 β-碳原子之间的距离来表征反应曲线。然而,Rotamerism 尚未得到广泛研究。在这里,我们使用高级量子化学计算,直到具有单、双和扰动三重激发 [CCSD(T)] 的耦合簇,以表征原型亲核试剂甲烷硫代酸盐对各种含氮 Michael 受体的亲核添加,这些受体是代表小分子共价抑制剂。通过研究所得烯醇化物的结构、能量和电子性质以及它们的反应曲线,我们表明,由于构象允许的有吸引力的非共价相互作用的幅度更大,因此在能量上通常比其他加成更有利。与加成过程相关的计算过渡态表明,与反平面加成相比,同步加成通过更低的能量屏障进行,并且是首选的反应途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号