当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In-Depth Mass Spectrometry Study of Vanadium(IV) Complexes with Model Peptides
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.inorgchem.4c02683
Kira Küssner 1 , Valeria Ugone 2 , Daniele Sanna 2 , Monika Cziferszky 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.inorgchem.4c02683
Kira Küssner 1 , Valeria Ugone 2 , Daniele Sanna 2 , Monika Cziferszky 1
Affiliation
|
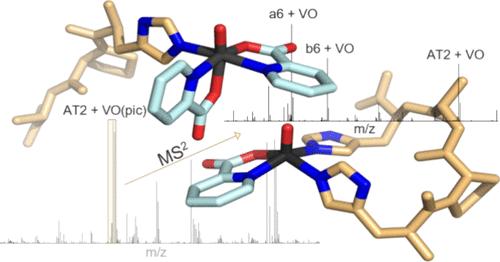
Investigating the speciation of vanadium complexes in the presence of potential biomolecular targets under physiological conditions remains challenging, and further experimental techniques are needed to better understand the mechanism of action of potential metallodrugs. The interaction of two model peptides (angiotensin I and angiotensin II) with three well-known oxidovanadium(IV) compounds with antidiabetic and/or anticancer activity, [VIVO(pic)2(H2O)], [VIVO(ma)2], and [VIVO(dhp)2] (where pic, ma, and dhp are picolinate, maltolate, and 1,2-dimethyl-3-hydroxy-4(1H)-pyridinonate anions, respectively), was investigated by ESI-MS/MS (electrospray ionization tandem mass spectrometry) and complemented by EPR (electron paramagnetic resonance) spectroscopy measurements and theoretical calculations at the DFT (density functional theory) level. The results demonstrated that vanadium–peptide bonds are preserved after HCD (higher energy collisional dissociation) fragmentation, allowing for the identification of binding sites through a detailed analysis of the fragmentation spectra. Angiotensin I (AT1) and angiotensin II (AT2) exhibited different coordination behaviors. AT1, with two His residues (His6, His9), prefers to form [AT1 + VOL] adducts with both histidine residues coordinated to the metal ion, while AT2, which has only His6, can bind the metal in a monodentate fashion, forming also [AT2 + VOL2] adducts. Insights from this study pave the way to ESI-MS/MS investigations of more complex systems, including target proteins and further development of vanadium-based drugs.
中文翻译:

钒 (IV) 与模型肽配合物的深入质谱研究
在生理条件下研究存在潜在生物分子靶标的钒配合物的形态仍然具有挑战性,需要进一步的实验技术来更好地了解潜在金属药物的作用机制。两种模型肽(血管紧张素 I 和血管紧张素 II)与三种众所周知的具有抗糖尿病和/或抗癌活性的氧化钒 (IV) 化合物的相互作用,[V IV O(pic) 2 (H 2 O)]、[V IV O (ma) 2 ]和[V IV O(dhp) 2 ](其中 pic、ma 和 dhp 分别是吡啶甲酸根、麦芽酸根和 1,2-二甲基-3-羟基-4(1H)-吡啶酸根阴离子)通过 ESI-MS/MS(电喷雾电离串联质谱)进行研究,并辅以 EPR(电子顺磁共振)光谱测量和 DFT(密度泛函理论)水平的理论计算。结果表明,在 HCD(高能碰撞解离)断裂后,钒-肽键得以保留,从而可以通过对断裂光谱的详细分析来识别结合位点。血管紧张素 I (AT1) 和血管紧张素 II (AT2) 表现出不同的协调行为。 AT1 具有两个 His 残基(His6、His9),更倾向于与两个组氨酸残基与金属离子配位形成 [AT1 + VOL] 加合物,而 AT2 仅具有 His6,可以以单齿方式结合金属,也形成[AT2 + VOL 2 ] 加合物。这项研究的见解为更复杂系统(包括目标蛋白和钒基药物的进一步开发)的 ESI-MS/MS 研究铺平了道路。
更新日期:2024-09-12
中文翻译:

钒 (IV) 与模型肽配合物的深入质谱研究
在生理条件下研究存在潜在生物分子靶标的钒配合物的形态仍然具有挑战性,需要进一步的实验技术来更好地了解潜在金属药物的作用机制。两种模型肽(血管紧张素 I 和血管紧张素 II)与三种众所周知的具有抗糖尿病和/或抗癌活性的氧化钒 (IV) 化合物的相互作用,[V IV O(pic) 2 (H 2 O)]、[V IV O (ma) 2 ]和[V IV O(dhp) 2 ](其中 pic、ma 和 dhp 分别是吡啶甲酸根、麦芽酸根和 1,2-二甲基-3-羟基-4(1H)-吡啶酸根阴离子)通过 ESI-MS/MS(电喷雾电离串联质谱)进行研究,并辅以 EPR(电子顺磁共振)光谱测量和 DFT(密度泛函理论)水平的理论计算。结果表明,在 HCD(高能碰撞解离)断裂后,钒-肽键得以保留,从而可以通过对断裂光谱的详细分析来识别结合位点。血管紧张素 I (AT1) 和血管紧张素 II (AT2) 表现出不同的协调行为。 AT1 具有两个 His 残基(His6、His9),更倾向于与两个组氨酸残基与金属离子配位形成 [AT1 + VOL] 加合物,而 AT2 仅具有 His6,可以以单齿方式结合金属,也形成[AT2 + VOL 2 ] 加合物。这项研究的见解为更复杂系统(包括目标蛋白和钒基药物的进一步开发)的 ESI-MS/MS 研究铺平了道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号