当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfidation of Nanoscale Zero-Valent Iron by Sulfide: The Dynamic Process, Mechanism, and Role of Ferrous Iron
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.est.4c04390 Wenqiang Xu 1 , Chenyun Xia 1 , Feng He 1, 2 , Zhenyu Wang 2 , Liyuan Liang 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.est.4c04390 Wenqiang Xu 1 , Chenyun Xia 1 , Feng He 1, 2 , Zhenyu Wang 2 , Liyuan Liang 3
Affiliation
|
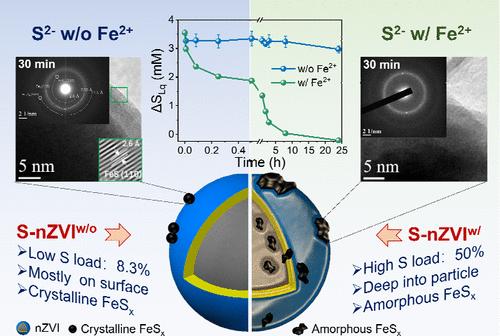
Sulfidation of nanoscale zerovalent iron (nZVI) can enhance particle performance. However, the underlying mechanisms of nZVI sulfidation are poorly known. We studied the effects of Fe2+ on 24-h dynamics of nZVI sulfidation by HS– using a dosed S to Fe molar ratio of 0.2. This shows that in the absence of Fe2+, HS– rapidly adsorbed onto nZVI particles and reacted with surface iron oxide to form mackinawite and greigite (<0.5 h). As nZVI corrosion progressed, amorphous FeSx in solution deposited on nZVI, forming S-nZVI (0.5–24 h). However, in the initial presence of Fe2+, the rapid reaction between HS– and Fe2+ produced amorphous FeSx, which deposited on the nZVI and corroded the surface iron oxide layer (<0.25 h). This was followed by redeposition of colloidal iron (hydr)oxide on the particle surface (0.25–8 h) and deposition of residual FeSx (8–24 h) on S-nZVI. S loading on S-nZVI was 1 order of magnitude higher when Fe2+ was present. Surface characterization of the sulfidated particles by TEM-SAED, XPS, and XAFS verified the solution dynamics and demonstrated that S2– and S22–/Sn2– were the principal reduced S species on S-nZVI. This study provides a methodology to tune sulfur loading and S speciation on S-nZVI to suit remediation needs.
中文翻译:

硫化物硫化纳米零价铁:二价铁的动态过程、机理和作用
纳米级零价铁 (nZVI) 的硫化可以增强颗粒性能。然而,nZVI 硫化的潜在机制尚不清楚。我们研究了 Fe 2+对 HS 硫化 nZVI 24 小时动力学的影响-使用剂量为 0.2 的 S 与 Fe 摩尔比。这表明,在没有Fe 2+的情况下,HS -快速吸附到nZVI颗粒上,并与表面氧化铁反应,形成硅镁石和镁灰石(<0.5 h)。随着nZVI腐蚀的进行,溶液中的非晶态FeS x沉积在nZVI上,形成S-nZVI(0.5-24小时)。然而,在Fe 2+初始存在的情况下,HS -和Fe 2+之间的快速反应产生非晶态FeS x ,其沉积在nZVI上并腐蚀表面氧化铁层(<0.25 h)。随后胶体氧化铁(氢)重新沉积在颗粒表面(0.25-8 小时),残留的 FeS x沉积(8-24 小时)在 S-nZVI 上。当存在 Fe 2+时,S-nZVI 上的 S 负载量高出 1 个数量级。通过 TEM-SAED、XPS 和 XAFS 对硫化颗粒进行表面表征,验证了溶液动力学,并证明 S 2–和 S 2 2– /S n 2–是 S-nZVI 上主要的还原 S 物种。本研究提供了一种调整 S-nZVI 上的硫负载和 S 形态以满足修复需求的方法。
更新日期:2024-09-12
中文翻译:

硫化物硫化纳米零价铁:二价铁的动态过程、机理和作用
纳米级零价铁 (nZVI) 的硫化可以增强颗粒性能。然而,nZVI 硫化的潜在机制尚不清楚。我们研究了 Fe 2+对 HS 硫化 nZVI 24 小时动力学的影响-使用剂量为 0.2 的 S 与 Fe 摩尔比。这表明,在没有Fe 2+的情况下,HS -快速吸附到nZVI颗粒上,并与表面氧化铁反应,形成硅镁石和镁灰石(<0.5 h)。随着nZVI腐蚀的进行,溶液中的非晶态FeS x沉积在nZVI上,形成S-nZVI(0.5-24小时)。然而,在Fe 2+初始存在的情况下,HS -和Fe 2+之间的快速反应产生非晶态FeS x ,其沉积在nZVI上并腐蚀表面氧化铁层(<0.25 h)。随后胶体氧化铁(氢)重新沉积在颗粒表面(0.25-8 小时),残留的 FeS x沉积(8-24 小时)在 S-nZVI 上。当存在 Fe 2+时,S-nZVI 上的 S 负载量高出 1 个数量级。通过 TEM-SAED、XPS 和 XAFS 对硫化颗粒进行表面表征,验证了溶液动力学,并证明 S 2–和 S 2 2– /S n 2–是 S-nZVI 上主要的还原 S 物种。本研究提供了一种调整 S-nZVI 上的硫负载和 S 形态以满足修复需求的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号