当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct construction of aryl amide N-glycosides from glycosyl oxamic acids via photoredox palladium-catalyzed aminocarbonylations
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-09-12 , DOI: 10.1016/j.checat.2024.101109 Xinyue Xie , Shiyin Zhao , Yang Han , Anrong Chen , Bo Yang , Bo Zhu , Yingzi Li , Jun Zhou , Feng Zhu
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-09-12 , DOI: 10.1016/j.checat.2024.101109 Xinyue Xie , Shiyin Zhao , Yang Han , Anrong Chen , Bo Yang , Bo Zhu , Yingzi Li , Jun Zhou , Feng Zhu
|
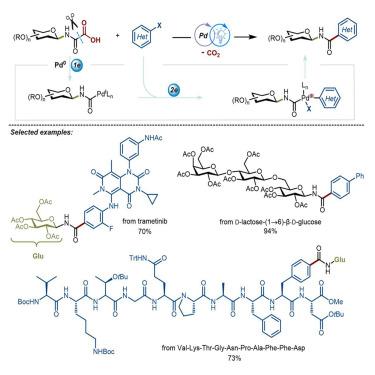
Despite the flourishing synthesis of O-glycosides, progress in N-glycoside synthesis has been impeded by significant challenges due to the weak nucleophilicity of amides. Here, we unveil an interesting photoredox palladium (Pd)-catalyzed aminocarbonylation of glycosyl oxamic acids with (hetero)aryl bromides to synthesize aryl N-amide glycosides. This method employs a merging single- and two-electron strategy for the first time, leveraging glycosyl oxamic acids as traceless carbamoyl radical precursors. By bypassing the elusive anomeric control of C–N glycosidic bond formation between sugars and aglycones, our approach offers a promising alternative for the synthesis of aryl amide N-glycosides. The versatility and applicability of this innovative strategy are demonstrated through a comprehensive examination of 65 examples, encompassing diverse (hetero)aryl electrophiles, saccharides, oligosaccharides, oligopeptides, and complex drug molecules. Mechanistic insights, gleaned from experimental and computational studies, elucidate a successive SET pathway and the generation of carbamoyl radicals in this synergistic catalytic process.
中文翻译:

通过光氧化钯催化的氨基羰基化反应,从糖基草酸直接构建芳基酰胺 N-糖苷
尽管 O-糖苷的合成蓬勃发展,但由于酰胺的亲核性较弱,N-糖苷合成的进展受到重大挑战的阻碍。在这里,我们揭示了一种有趣的光氧化还原钯 (Pd) 催化的糖基草酸与(杂)芳基溴的氨基羰基化反应,以合成芳基 N-酰胺糖苷。该方法首次采用了合并单电子和双电子策略,利用糖基草酸作为无痕量氨基甲酰自由基前体。通过绕过糖和糖苷配基之间 C-N 糖苷键形成的难以捉摸的异头控制,我们的方法为芳基酰胺 N-糖苷的合成提供了一种有前途的替代方案。通过对 65 个实例的全面检查,包括不同的(异)芳基亲电试剂、糖类、低聚糖、寡肽和复杂的药物分子,证明了这种创新策略的多功能性和适用性。从实验和计算研究中收集的机理见解阐明了连续的 SET 途径和该协同催化过程中氨基甲酰自由基的产生。
更新日期:2024-09-12
中文翻译:

通过光氧化钯催化的氨基羰基化反应,从糖基草酸直接构建芳基酰胺 N-糖苷
尽管 O-糖苷的合成蓬勃发展,但由于酰胺的亲核性较弱,N-糖苷合成的进展受到重大挑战的阻碍。在这里,我们揭示了一种有趣的光氧化还原钯 (Pd) 催化的糖基草酸与(杂)芳基溴的氨基羰基化反应,以合成芳基 N-酰胺糖苷。该方法首次采用了合并单电子和双电子策略,利用糖基草酸作为无痕量氨基甲酰自由基前体。通过绕过糖和糖苷配基之间 C-N 糖苷键形成的难以捉摸的异头控制,我们的方法为芳基酰胺 N-糖苷的合成提供了一种有前途的替代方案。通过对 65 个实例的全面检查,包括不同的(异)芳基亲电试剂、糖类、低聚糖、寡肽和复杂的药物分子,证明了这种创新策略的多功能性和适用性。从实验和计算研究中收集的机理见解阐明了连续的 SET 途径和该协同催化过程中氨基甲酰自由基的产生。































 京公网安备 11010802027423号
京公网安备 11010802027423号