当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu3VSe4 Cathode for Rechargeable Magnesium Batteries: Favorable Chemical and Electronic Structures Inducing Intercalation and Displacement Reactions
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-09-12 , DOI: 10.1002/adfm.202411223 Donggang Tao 1 , Ting Li 2 , Yudi Tang 1 , Hongda Gui 1 , Yuliang Cao 3 , Fei Xu 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-09-12 , DOI: 10.1002/adfm.202411223 Donggang Tao 1 , Ting Li 2 , Yudi Tang 1 , Hongda Gui 1 , Yuliang Cao 3 , Fei Xu 1
Affiliation
|
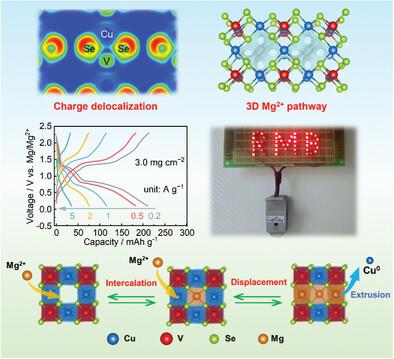
Rechargeable Mg batteries are an advantageous energy-storage technology with low cost and high safety, but the design of high-performance cathode materials is currently the major difficulty. Herein, a new cathode material of Cu3VSe4 is fabricated with a comprehensive consideration of the chemical and electronic structures. The intermediate band semiconductor Cu3VSe4 has a cubic crystal structure containing interlaced 3D tunnels. The V and Se atoms form chemical bonds with high covalent proportions and facilitate the charge delocalization via the V‒Se bonds. Because of these features, Cu3VSe4 provides a high capacity of 251 mAh g‒1 with co-redox of Cu, V, and Se elements and an outstanding rate performance of 44 mAh g‒1 at 15 A g‒1. Prominently, a high mass load of 3.0 mg cm‒2 is achieved without obvious rate capability decay, which is quite favorable to pair with the high-capacity Mg metal anode in practical application. The mechanism investigation and theoretical computation demonstrate that Cu3VSe4 undergoes first a Mg-intercalation and then a displacement reaction, during which the crystal structure is maintained, assisting the reaction reversibility and cycling stability. These findings reveal a rational design principle of rechargeable Mg battery cathodes based on a comprehensive consideration of chemical and electronic structures.
中文翻译:

用于可充电镁电池的 Cu3VSe4 阴极:诱导插层和置换反应的有利化学和电子结构
可充电镁电池是一种成本低、安全性高的有利储能技术,但高性能正极材料的设计是目前的主要难点。在此,在综合考虑化学和电子结构的情况下,制备了一种新型的 Cu3VSe4 正极材料。中间带半导体 Cu3VSe4 具有包含交错 3D 隧道的立方晶体结构。V 和 Se 原子形成高共价比例的化学键,并通过 V\u2012Se 键促进电荷离域。由于这些特性,Cu3VSe4 提供 251 mAh g\u20121 的高容量,具有 Cu、V 和 Se 元素的共氧化还原,以及在 15 A g\u20121 时 44 mAh g\u20121 的出色倍率性能。突出的是,实现了 3.0 mg cm\u20122 的高质量负载,没有明显的倍率能力衰减,这非常有利于在实际应用中与高容量 Mg 金属负极配对。机理研究和理论计算表明,Cu3VSe4 首先经历 Mg-插层反应,然后发生置换反应,在此期间保持晶体结构,有助于反应可逆性和循环稳定性。这些发现揭示了基于化学和电子结构综合考虑的可充电镁电池正极的合理设计原则。
更新日期:2024-09-12
中文翻译:

用于可充电镁电池的 Cu3VSe4 阴极:诱导插层和置换反应的有利化学和电子结构
可充电镁电池是一种成本低、安全性高的有利储能技术,但高性能正极材料的设计是目前的主要难点。在此,在综合考虑化学和电子结构的情况下,制备了一种新型的 Cu3VSe4 正极材料。中间带半导体 Cu3VSe4 具有包含交错 3D 隧道的立方晶体结构。V 和 Se 原子形成高共价比例的化学键,并通过 V\u2012Se 键促进电荷离域。由于这些特性,Cu3VSe4 提供 251 mAh g\u20121 的高容量,具有 Cu、V 和 Se 元素的共氧化还原,以及在 15 A g\u20121 时 44 mAh g\u20121 的出色倍率性能。突出的是,实现了 3.0 mg cm\u20122 的高质量负载,没有明显的倍率能力衰减,这非常有利于在实际应用中与高容量 Mg 金属负极配对。机理研究和理论计算表明,Cu3VSe4 首先经历 Mg-插层反应,然后发生置换反应,在此期间保持晶体结构,有助于反应可逆性和循环稳定性。这些发现揭示了基于化学和电子结构综合考虑的可充电镁电池正极的合理设计原则。































 京公网安备 11010802027423号
京公网安备 11010802027423号