当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimized Enzyme Production for the Escherichia coli Whole-Cell Biocatalytic Synthesis of Codeine from Thebaine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.oprd.4c00195 Ali Jahanian 1, 2, 3 , Andres Velasquez Agudelo 4 , Carlos Horacio Luna-Flores 2, 4 , Xu Li 5, 6 , Fiona Fry 7 , George Mutch 7 , Geoffrey W. Stevens 5 , Sally L. Gras 5, 6 , Robert E. Speight 2, 3, 4, 8
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.oprd.4c00195 Ali Jahanian 1, 2, 3 , Andres Velasquez Agudelo 4 , Carlos Horacio Luna-Flores 2, 4 , Xu Li 5, 6 , Fiona Fry 7 , George Mutch 7 , Geoffrey W. Stevens 5 , Sally L. Gras 5, 6 , Robert E. Speight 2, 3, 4, 8
Affiliation
|
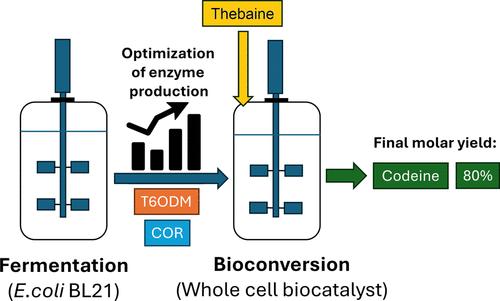
Codeine, the most widely used medicinal opiate in the world, can be produced from thebaine by using an environmentally friendly biocatalytic process using purified enzymes or microbial cells. However, this biotransformation is either inefficient or not systematically characterized for industrial use, as microbial fermentation and enzyme expression have not been optimized, and the production of the undesirable side product neopine reduces yields. In this study, the expression of the required enzymes in a reengineeredEscherichia coli system was optimized using a two-step design of experiments approach. Higher yields of biomass and enzyme expression were achieved in a defined minimal medium using lactose for induction. The interaction between temperature and lactose concentration was optimized, leading to an improvement in the apparent volumetric activity of thebaine 6-O-demethylase (T6ODM) from 45 to 776 U·L–1 using glycerol as the carbon source and an improvement in the apparent volumetric activity of codeinone reductase (COR) from 120 to 3707 U·L–1 using glucose. The productivity for T6ODM and COR was also increased 4.5 and 5.7-times to 8.0 and 28.3 U·L–1·h–1, respectively, in fed-batch cultivations compared to in shake flasks. With the optimized conditions for enzyme expression, the whole-cell biotransformation led to an 80% yield of codeine from thebaine and a final codeine/neopine molar ratio of 85:15. The inclusion of coexpressed neopinone isomerase, which catalyzes the production of codeinone from neopinone, reduced neopine production at the start of the reaction when the concentration of neopinone was low but did not improve the final codeine/neopine ratio as the reaction progressed and the concentration of neopinone increased. This observation may provide a further understanding of the differences between catalytic conditions in a bioreactor and the poppy plant, where the concentration of pathway intermediates such as neopinone is likely to be low and no neopine is observed. Overall, the process improvements reported here provide progress toward an industrial biocatalytic production of codeine from thebaine.
中文翻译:

大肠杆菌全细胞生物催化从蒂巴因合成可待因的优化酶生产
可待因是世界上使用最广泛的药用阿片剂,可以通过使用纯化的酶或微生物细胞的环保生物催化工艺从蒂巴因生产。然而,这种生物转化要么效率低下,要么没有系统地表征用于工业用途,因为微生物发酵和酶表达尚未优化,并且不良副产物新平的产生降低了产量。在本研究中,使用两步实验设计方法优化了重新设计的大肠杆菌系统中所需酶的表达。使用乳糖进行诱导,在确定的基本培养基中实现了更高的生物量和酶表达产量。优化了温度和乳糖浓度之间的相互作用,使用甘油作为碳源,使蒂巴因 6 -O-脱甲基酶 (T6ODM) 的表观体积活性从 45 U·L –1 提高到 776 U·L –1 ,并提高了表观体积活性。使用葡萄糖时,可待因酮还原酶 (COR) 的体积活性从 120 U·L –1 提高到 3707 U·L –1 。与摇瓶培养相比,分批补料培养中 T6ODM 和 COR 的生产率也分别提高了 4.5 倍和 5.7 倍,分别达到 8.0 和 28.3 U·L –1 ·h –1 。通过优化酶表达条件,全细胞生物转化从蒂巴因中获得可待因的产率为 80%,最终可待因/新安品摩尔比为 85:15。 共表达的尼奥品酮异构酶催化从尼奥品酮产生可待因酮,当尼奥品酮浓度较低时,在反应开始时减少了尼奥品的产生,但随着反应的进行和浓度的增加,最终的可待因/尼奥品比率没有提高。尼奥品酮增加。这一观察结果可以提供对生物反应器和罂粟植物中催化条件之间差异的进一步了解,其中途径中间体(例如新平酮)的浓度可能较低并且没有观察到新平。总体而言,本文报道的工艺改进为从蒂巴因工业生物催化生产可待因提供了进展。
更新日期:2024-09-12
中文翻译:

大肠杆菌全细胞生物催化从蒂巴因合成可待因的优化酶生产
可待因是世界上使用最广泛的药用阿片剂,可以通过使用纯化的酶或微生物细胞的环保生物催化工艺从蒂巴因生产。然而,这种生物转化要么效率低下,要么没有系统地表征用于工业用途,因为微生物发酵和酶表达尚未优化,并且不良副产物新平的产生降低了产量。在本研究中,使用两步实验设计方法优化了重新设计的大肠杆菌系统中所需酶的表达。使用乳糖进行诱导,在确定的基本培养基中实现了更高的生物量和酶表达产量。优化了温度和乳糖浓度之间的相互作用,使用甘油作为碳源,使蒂巴因 6 -O-脱甲基酶 (T6ODM) 的表观体积活性从 45 U·L –1 提高到 776 U·L –1 ,并提高了表观体积活性。使用葡萄糖时,可待因酮还原酶 (COR) 的体积活性从 120 U·L –1 提高到 3707 U·L –1 。与摇瓶培养相比,分批补料培养中 T6ODM 和 COR 的生产率也分别提高了 4.5 倍和 5.7 倍,分别达到 8.0 和 28.3 U·L –1 ·h –1 。通过优化酶表达条件,全细胞生物转化从蒂巴因中获得可待因的产率为 80%,最终可待因/新安品摩尔比为 85:15。 共表达的尼奥品酮异构酶催化从尼奥品酮产生可待因酮,当尼奥品酮浓度较低时,在反应开始时减少了尼奥品的产生,但随着反应的进行和浓度的增加,最终的可待因/尼奥品比率没有提高。尼奥品酮增加。这一观察结果可以提供对生物反应器和罂粟植物中催化条件之间差异的进一步了解,其中途径中间体(例如新平酮)的浓度可能较低并且没有观察到新平。总体而言,本文报道的工艺改进为从蒂巴因工业生物催化生产可待因提供了进展。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号