当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Deoxygenative [3+2] Cycloaddition of N-Hydroxyamides with Alkynes to Access Isoxazoles
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.orglett.4c02852 Haixiang Wang 1 , Yan Sun 1 , Wentong Liu 1 , Liliang Huang 1 , Huangdi Feng 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.orglett.4c02852 Haixiang Wang 1 , Yan Sun 1 , Wentong Liu 1 , Liliang Huang 1 , Huangdi Feng 1
Affiliation
|
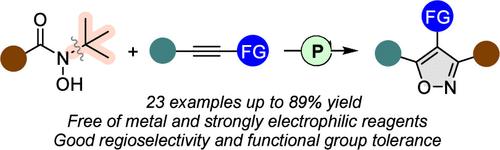
Although the transition-metal-catalyzed [3+2] cycloadditions to access isoxazoles have been described well, organocatalytic methods remain underdeveloped. Herein, we report the use of an organophosphine catalyst for the preparation of a series of isoxazoles with exceptional regioselectivity via the [3+2] cycloaddition of N-hydroxyamides and alkynes. The scope of this organocatalytic transformation is broad, tolerating numerous functional groups and proceeding uniformly in an environmentally friendly, simple, and efficient manner.
中文翻译:

N-羟基酰胺与炔烃的有机催化脱氧[3+2]环加成反应生成异恶唑
尽管过渡金属催化的[3+2]环加成反应获得异恶唑已经得到很好的描述,但有机催化方法仍然不发达。在此,我们报道了使用有机膦催化剂通过N-羟基酰胺和炔烃的[3+2]环加成制备一系列具有优异区域选择性的异恶唑。这种有机催化转化的范围很广,可以容纳多种官能团,并且以环境友好、简单、高效的方式均匀地进行。
更新日期:2024-09-12
中文翻译:

N-羟基酰胺与炔烃的有机催化脱氧[3+2]环加成反应生成异恶唑
尽管过渡金属催化的[3+2]环加成反应获得异恶唑已经得到很好的描述,但有机催化方法仍然不发达。在此,我们报道了使用有机膦催化剂通过N-羟基酰胺和炔烃的[3+2]环加成制备一系列具有优异区域选择性的异恶唑。这种有机催化转化的范围很广,可以容纳多种官能团,并且以环境友好、简单、高效的方式均匀地进行。































 京公网安备 11010802027423号
京公网安备 11010802027423号