当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Converting Strain Release into Aromaticity Loss for Activation of Donor–Acceptor Cyclopropanes: Generation of Quinone Methide Traps for C-Nucleophiles
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.orglett.4c03106 Vitaly V Shorokhov 1 , Beauty K Chabuka 2 , Timur P Tikhonov 1 , Anastasia V Filippova 1 , Sergey S Zhokhov 1 , Victor A Tafeenko 1 , Ivan A Andreev 3 , Nina K Ratmanova 3 , Maxim G Uchuskin 4 , Igor V Trushkov 5 , Igor V Alabugin 2 , Olga A Ivanova 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.orglett.4c03106 Vitaly V Shorokhov 1 , Beauty K Chabuka 2 , Timur P Tikhonov 1 , Anastasia V Filippova 1 , Sergey S Zhokhov 1 , Victor A Tafeenko 1 , Ivan A Andreev 3 , Nina K Ratmanova 3 , Maxim G Uchuskin 4 , Igor V Trushkov 5 , Igor V Alabugin 2 , Olga A Ivanova 1
Affiliation
|
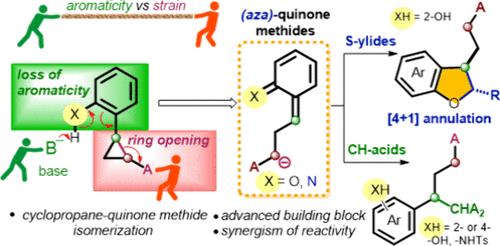
Here, we present a new approach for the activation of donor–acceptor cyclopropanes in ring-opening reactions, which does not require the use of a Lewis or Brønsted acid as a catalyst. Donor–acceptor cyclopropanes containing a phenolic group as the donor undergo deprotonation and isomerization to form the corresponding quinone methides. This innovative strategy was applied to achieve (4 + 1)-annulation of cyclopropanes with sulfur ylides, affording functionalized dihydrobenzofurans. Additionally, the generated ortho- and para-(aza)quinone methides can be trapped by various CH-acids.
中文翻译:

将应变释放转化为芳香度损失以激活供体-受体环丙烷:生成 C-亲核试剂的醌甲基化物陷阱
在这里,我们提出了一种在开环反应中活化供体-受体环丙烷的新方法,该方法不需要使用路易斯酸或布朗斯台德酸作为催化剂。含有酚基作为供体的供体-受体环丙烷进行去质子化和异构化,形成相应的醌甲基化物。这一创新策略用于实现环丙烷与硫叶立德的 (4 + 1) 环化,得到功能化的二氢苯并呋喃。此外,生成的邻-和对-(氮杂)醌甲基化物可以被各种CH-酸捕获。
更新日期:2024-09-12
中文翻译:

将应变释放转化为芳香度损失以激活供体-受体环丙烷:生成 C-亲核试剂的醌甲基化物陷阱
在这里,我们提出了一种在开环反应中活化供体-受体环丙烷的新方法,该方法不需要使用路易斯酸或布朗斯台德酸作为催化剂。含有酚基作为供体的供体-受体环丙烷进行去质子化和异构化,形成相应的醌甲基化物。这一创新策略用于实现环丙烷与硫叶立德的 (4 + 1) 环化,得到功能化的二氢苯并呋喃。此外,生成的邻-和对-(氮杂)醌甲基化物可以被各种CH-酸捕获。































 京公网安备 11010802027423号
京公网安备 11010802027423号