当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand-Enabled Copper-Catalyzed Ullmann-Type S–C Bond Formation to Access Sulfilimines
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.orglett.4c03116 Xianda Wu 1 , Jiayi Zheng 1 , Fu-Sheng He 1 , Jie Wu 1, 2, 3
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-12 , DOI: 10.1021/acs.orglett.4c03116 Xianda Wu 1 , Jiayi Zheng 1 , Fu-Sheng He 1 , Jie Wu 1, 2, 3
Affiliation
|
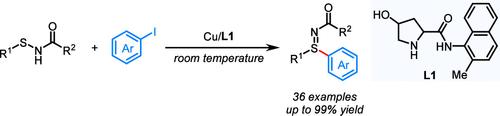
A copper-catalyzed Ullmann-type cross-coupling reaction of sulfenamides with aryl iodides is developed. The key to success is the use of a 2-methylnaphthalen-1-amine-derived amide ligand, which enables the formation of an S–C bond to access functionalized sulfilimines in good to excellent yields at room temperature. This method has the advantages of mild conditions, a broad substrate scope, good functional group compatibility, and high chemoselectivity. The utility of this protocol is highlighted through late-stage modification of drug-relevant molecules and sulfilimine product derivatization.
中文翻译:

配体铜催化乌尔曼型 S-C 键形成以获得硫亚胺
开发了铜催化的次磺酰胺与芳基碘化物的乌尔曼型交叉偶联反应。成功的关键是使用 2-甲基萘-1-胺衍生的酰胺配体,它能够形成 S-C 键,从而在室温下以良好至优异的产率获得官能化硫亚胺。该方法具有条件温和、底物范围广、官能团相容性好、化学选择性高等优点。该协议的实用性通过药物相关分子的后期修饰和硫亚胺产品衍生化得到强调。
更新日期:2024-09-12
中文翻译:

配体铜催化乌尔曼型 S-C 键形成以获得硫亚胺
开发了铜催化的次磺酰胺与芳基碘化物的乌尔曼型交叉偶联反应。成功的关键是使用 2-甲基萘-1-胺衍生的酰胺配体,它能够形成 S-C 键,从而在室温下以良好至优异的产率获得官能化硫亚胺。该方法具有条件温和、底物范围广、官能团相容性好、化学选择性高等优点。该协议的实用性通过药物相关分子的后期修饰和硫亚胺产品衍生化得到强调。































 京公网安备 11010802027423号
京公网安备 11010802027423号