Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sample-to-answer centrifugal microfluidic droplet PCR platform for quantitation of viral load
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4lc00533c Lidija Malic , Liviu Clime , Byeong-Ui Moon , Christina Nassif , Dillon Da Fonte , Daniel Brassard , Ljuboje Lukic , Matthias Geissler , Keith J Morton , Denis Charlebois , Teodor Veres
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4lc00533c Lidija Malic , Liviu Clime , Byeong-Ui Moon , Christina Nassif , Dillon Da Fonte , Daniel Brassard , Ljuboje Lukic , Matthias Geissler , Keith J Morton , Denis Charlebois , Teodor Veres
|
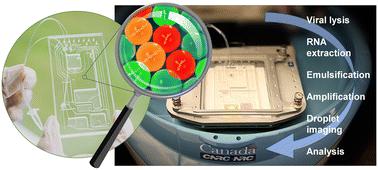
Droplet digital polymerase chain reaction (ddPCR) stands out as a highly sensitive diagnostic technique that is gaining traction in infectious disease diagnostics due to its ability to quantitate very low numbers of viral gene copies. By partitioning the sample into thousands of droplets, ddPCR enables precise and absolute quantification without relying on a standard curve. However, current ddPCR systems often exhibit relatively low levels of integration, and the analytical process remains dependent on elaborate workflows for up-front sample preparation. Here, we introduce a fully-integrated system seamlessly combining viral lysis, RNA extraction, emulsification, reverse transcription (RT) ddPCR, and fluorescence readout in a sample-to-answer format. The system comprises a disposable microfluidic cartridge housing buffers and reagents required for the assay, and a centrifugal platform that allows for pneumatic actuation of liquids during rotation, enabling automation of the workflow. Highly monodisperse droplets (∼50 μm in diameter) are produced using centrifugal step emulsification and automatically transferred to an integrated heating module for target amplification. The platform is equipped with a miniature fluorescence imaging system enabling on-chip read-out of droplets after RT-ddPCR. We demonstrate sample-to-answer detection of SARS-CoV-2 N and E genes, along with RNase P endogenous reference, using hydrolysis probes and multiplexed amplification within single droplets for concentrations as low as 0.1 copy per μL. We also tested 14 nasopharyngeal swab specimens from patients and were able to distinguish positive and negative SARS-CoV-2 samples with 100% accuracy, surpassing results obtained by conventional real-time amplification.
中文翻译:

用于病毒载量定量的 Samp-to-Answer 离心微流控液滴 PCR 平台
液滴数字聚合酶链反应 (ddPCR) 是一种高度敏感的诊断技术,由于其能够定量非常少量的病毒基因拷贝,因此在传染病诊断中越来越受欢迎。通过将样品分成数千个液滴,ddPCR 无需依赖标准曲线即可实现精确和绝对的定量。然而,当前的 ddPCR 系统通常表现出相对较低的集成水平,并且分析过程仍然依赖于复杂的前期样品制备工作流程。在这里,我们介绍了一个完全集成的系统,该系统以样本到结果的形式无缝地结合了病毒裂解、RNA 提取、乳化、逆转录 (RT) ddPCR 和荧光读数。该系统包括一个一次性微流体卡盒,其中装有分析所需的缓冲液和试剂,以及一个离心平台,允许在旋转过程中对液体进行气动驱动,从而实现工作流程的自动化。使用离心步法乳化产生高度单分散的液滴(直径 ∼50 μm),并自动转移到集成的加热模块进行靶标扩增。该平台配备了微型荧光成像系统,可在 RT-ddPCR 后在芯片上读取液滴。我们展示了使用水解探针和单液滴内多重扩增(浓度低至 0.1 拷贝/μL)对 SARS-CoV-2 N 和 E 基因以及 RNase P 内源性参比的样本到结果检测。我们还测试了 14 份患者的鼻咽拭子样本,能够以 100% 的准确率区分阳性和阴性 SARS-CoV-2 样本,超过了传统实时扩增获得的结果。
更新日期:2024-09-11
中文翻译:

用于病毒载量定量的 Samp-to-Answer 离心微流控液滴 PCR 平台
液滴数字聚合酶链反应 (ddPCR) 是一种高度敏感的诊断技术,由于其能够定量非常少量的病毒基因拷贝,因此在传染病诊断中越来越受欢迎。通过将样品分成数千个液滴,ddPCR 无需依赖标准曲线即可实现精确和绝对的定量。然而,当前的 ddPCR 系统通常表现出相对较低的集成水平,并且分析过程仍然依赖于复杂的前期样品制备工作流程。在这里,我们介绍了一个完全集成的系统,该系统以样本到结果的形式无缝地结合了病毒裂解、RNA 提取、乳化、逆转录 (RT) ddPCR 和荧光读数。该系统包括一个一次性微流体卡盒,其中装有分析所需的缓冲液和试剂,以及一个离心平台,允许在旋转过程中对液体进行气动驱动,从而实现工作流程的自动化。使用离心步法乳化产生高度单分散的液滴(直径 ∼50 μm),并自动转移到集成的加热模块进行靶标扩增。该平台配备了微型荧光成像系统,可在 RT-ddPCR 后在芯片上读取液滴。我们展示了使用水解探针和单液滴内多重扩增(浓度低至 0.1 拷贝/μL)对 SARS-CoV-2 N 和 E 基因以及 RNase P 内源性参比的样本到结果检测。我们还测试了 14 份患者的鼻咽拭子样本,能够以 100% 的准确率区分阳性和阴性 SARS-CoV-2 样本,超过了传统实时扩增获得的结果。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号