当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective transition metal-catalyzed [(2+2)+1] and [(2+2)+2] carbocyclization reactions using 1,6-enynes with 1,1-disubstituted olefins: construction of quaternary centers
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-12 , DOI: 10.1039/d4sc02645d Ridge Michael P. Ylagan , Yu Zhu , P. Andrew Evans
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-12 , DOI: 10.1039/d4sc02645d Ridge Michael P. Ylagan , Yu Zhu , P. Andrew Evans
|
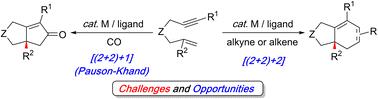
Transition metal-catalyzed carbocyclization reactions provide a powerful method for the stereoselective assembly of complex, highly substituted (poly)cyclic scaffolds. Although 1,6-enynes are common substrates for these transformations, using polysubstituted alkene derivatives to construct functionalized cyclic products remains challenging due to their significantly lower reactivity. This Perspective highlights key developments in stereoselective semi-intramolecular metal-catalyzed [(2+2)+1] and [(2+2)+2] carbocyclizations of 1,6-enynes containing 1,1-disubstituted alkenes, which produce cycloadducts with quaternary stereogenic centers. The insights gleaned from these examples provide a blueprint for developing more general carbocyclization strategies with challenging polysubstituted olefins.
中文翻译:

使用 1,6-烯炔和 1,1-二取代烯烃的立体选择性过渡金属催化的 [(2+2)+1] 和 [(2+2)+2] 碳环化反应:四元中心的构建
过渡金属催化的碳环化反应为复杂、高度取代(多)环支架的立体选择性组装提供了一种强大的方法。尽管 1,6-烯炔是这些转化的常见底物,但由于它们的反应性明显较低,使用多取代烯烃衍生物构建功能化环状产物仍然具有挑战性。该观点强调了立体选择性半分子内金属催化的 [(2+2)+1] 和 [(2+2)+2] 含有 1,1-二取代烯烃的 1,6-烯烃的碳环化的关键发展,这些烯烃产生具有四元立体中心的环加合物。从这些例子中收集的见解为使用具有挑战性的聚乙烯取代烯烃开发更通用的碳环化策略提供了蓝图。
更新日期:2024-09-12
中文翻译:

使用 1,6-烯炔和 1,1-二取代烯烃的立体选择性过渡金属催化的 [(2+2)+1] 和 [(2+2)+2] 碳环化反应:四元中心的构建
过渡金属催化的碳环化反应为复杂、高度取代(多)环支架的立体选择性组装提供了一种强大的方法。尽管 1,6-烯炔是这些转化的常见底物,但由于它们的反应性明显较低,使用多取代烯烃衍生物构建功能化环状产物仍然具有挑战性。该观点强调了立体选择性半分子内金属催化的 [(2+2)+1] 和 [(2+2)+2] 含有 1,1-二取代烯烃的 1,6-烯烃的碳环化的关键发展,这些烯烃产生具有四元立体中心的环加合物。从这些例子中收集的见解为使用具有挑战性的聚乙烯取代烯烃开发更通用的碳环化策略提供了蓝图。































 京公网安备 11010802027423号
京公网安备 11010802027423号