当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Consecutive chirality transfer: efficient synthesis of chiral C,O-chelated BINOL/gold(III) complexes for asymmetric catalysis and chiral resolution of disubstituted BINOLs
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-12 , DOI: 10.1039/d4sc04221b Kwok-Heung Aries Chan , Wa-Yi O , Jia-Jun Jiang , Jian-Fang Cui , Man Kin Wong
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-12 , DOI: 10.1039/d4sc04221b Kwok-Heung Aries Chan , Wa-Yi O , Jia-Jun Jiang , Jian-Fang Cui , Man Kin Wong
|
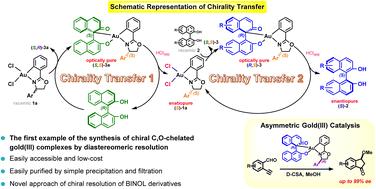
A novel approach for efficient synthesis of chiral C,O-chelated BINOL/gold(III) complexes by diastereomeric resolution using enantiopure BINOL as a chiral resolving agent was demonstrated. The BINOL/gold(III) diastereomers with different solubility were separated by simple filtration, providing optically pure BINOL/gold(III) complexes with up to >99 : 1 dr. By combining this with an efficient BINOL ligand dissociation process, a simple and column-free method for chiral resolution of racemic gold(III) dichloride complexes on a gram scale was established, affording their enantiopure forms in good yields. Conversely, the resolved enantiopure gold(III) dichloride complexes could serve as chiral resolving agents to resolve disubstituted BINOL derivatives, achieving both BINOLs and gold(III) complexes in good to excellent yields (overall 77–96% and 76–95%, respectively) with a high optical purity of up to 99% ee. Through a consecutive chirality transfer process, the chiral information from an inexpensive chiral source was transferred to highly valuable gold(III) complexes, followed by sterically bulky BINOL derivatives. This work would open a new synthetic strategy facilitating the development of structurally diverse chiral gold(III) complexes and gold(III)-mediated chiral resolution of BINOL derivatives. In addition, this new class of C,O-chelated BINOL/gold(III) complexes achieved asymmetric carboalkoxylation of ortho-alkynylbenzaldehydes with an excellent enantioselectivity of up to 99% ee.
中文翻译:

连续手性转移:有效合成手性 C,O-螯合 BINOL/金 (III) 配合物,用于不对称催化和二取代 BINOL 的手性拆分
展示了一种使用对映纯BINOL作为手性拆分剂通过非对映体拆分有效合成手性C,O-螯合BINOL/金( III )配合物的新方法。通过简单过滤分离具有不同溶解度的BINOL/金( III )非对映异构体,提供高达>99:1dr的光学纯BINOL/金( III )复合物。通过将其与有效的 BINOL 配体解离过程相结合,建立了一种简单且无柱的方法,用于克级规模的外消旋二氯化金 ( III ) 络合物的手性拆分,并以良好的产率提供其对映体纯形式。相反,拆分的对映纯二氯化金( III )配合物可以作为手性拆分剂来拆分双取代的BINOL衍生物,以良好至优异的产率获得BINOL和金( III )配合物(总体分别为77-96%和76-95%) )具有高达 99% ee 的高光学纯度。通过连续的手性转移过程,来自廉价手性源的手性信息被转移到高价值的金( III )络合物,然后是空间庞大的联醇衍生物。这项工作将开辟一种新的合成策略,促进结构多样化的手性金 ( III ) 配合物的开发和金 ( III ) 介导的 BINOL 衍生物的手性拆分。此外,这种新型C,O-螯合BINOL/金( III )配合物实现了邻炔基苯甲醛的不对称烷氧基化,具有高达99% ee的优异对映选择性。
更新日期:2024-09-12
中文翻译:

连续手性转移:有效合成手性 C,O-螯合 BINOL/金 (III) 配合物,用于不对称催化和二取代 BINOL 的手性拆分
展示了一种使用对映纯BINOL作为手性拆分剂通过非对映体拆分有效合成手性C,O-螯合BINOL/金( III )配合物的新方法。通过简单过滤分离具有不同溶解度的BINOL/金( III )非对映异构体,提供高达>99:1dr的光学纯BINOL/金( III )复合物。通过将其与有效的 BINOL 配体解离过程相结合,建立了一种简单且无柱的方法,用于克级规模的外消旋二氯化金 ( III ) 络合物的手性拆分,并以良好的产率提供其对映体纯形式。相反,拆分的对映纯二氯化金( III )配合物可以作为手性拆分剂来拆分双取代的BINOL衍生物,以良好至优异的产率获得BINOL和金( III )配合物(总体分别为77-96%和76-95%) )具有高达 99% ee 的高光学纯度。通过连续的手性转移过程,来自廉价手性源的手性信息被转移到高价值的金( III )络合物,然后是空间庞大的联醇衍生物。这项工作将开辟一种新的合成策略,促进结构多样化的手性金 ( III ) 配合物的开发和金 ( III ) 介导的 BINOL 衍生物的手性拆分。此外,这种新型C,O-螯合BINOL/金( III )配合物实现了邻炔基苯甲醛的不对称烷氧基化,具有高达99% ee的优异对映选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号