当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A homoleptic Fe(IV) ketimide complex with a low-lying excited state
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-10 , DOI: 10.1039/d4sc04880f Phoebe Hertler , Trevor W. Hayton , Arturo Sauza-de la Vega , Andrea Daru , Arup Sarkar , Richard A. Lewis , Guang Wu , Laura Gagliardi
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-10 , DOI: 10.1039/d4sc04880f Phoebe Hertler , Trevor W. Hayton , Arturo Sauza-de la Vega , Andrea Daru , Arup Sarkar , Richard A. Lewis , Guang Wu , Laura Gagliardi
|
The reaction of 4 equiv. of Li(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph) with FeIICl2 results in isolation of [Li(Et2O)]2[FeII(N
C(tBu)Ph) with FeIICl2 results in isolation of [Li(Et2O)]2[FeII(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph)4] (1), in good yields. The reaction of 1 with 1 equiv. of I2 leads to formation of [FeIV(N
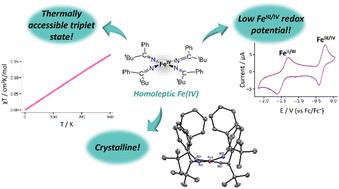
C(tBu)Ph)4] (1), in good yields. The reaction of 1 with 1 equiv. of I2 leads to formation of [FeIV(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph)4] (2), in moderate yields. 57Fe Mössbauer spectroscopy confirms the Fe(IV) oxidation state of 2, and X-ray crystallography reveals that 2 has a square planar coordination geometry along with several intramolecular H⋯C interactions. Furthermore, SQUID magnetometry indicates a small magnetic moment at room temperature, suggestive of an accessible S = 1 state. Both density functional theory and multiconfigurational calculations were done to elucidate the nature of the ground state. Consistent with the experimental results, the ground state was found to be an S = 0 state with an S = 1 excited state close in energy.
C(tBu)Ph)4] (2), in moderate yields. 57Fe Mössbauer spectroscopy confirms the Fe(IV) oxidation state of 2, and X-ray crystallography reveals that 2 has a square planar coordination geometry along with several intramolecular H⋯C interactions. Furthermore, SQUID magnetometry indicates a small magnetic moment at room temperature, suggestive of an accessible S = 1 state. Both density functional theory and multiconfigurational calculations were done to elucidate the nature of the ground state. Consistent with the experimental results, the ground state was found to be an S = 0 state with an S = 1 excited state close in energy.
中文翻译:

具有低位激发态的同源 Fe(IV) 酮类络合物
4 当量的 Li(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph)) 与 FeIICl2 反应导致分离出 [Li(Et2O)]2[FeII(N
C(tBu)Ph)) 与 FeIICl2 反应导致分离出 [Li(Et2O)]2[FeII(N ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph)4] (1),收率良好。1 与 1 当量的 I2 反应导致形成 [FeIV(N
C(tBu)Ph)4] (1),收率良好。1 与 1 当量的 I2 反应导致形成 [FeIV(N ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph)4] (2),产率适中。57 元Fe Mössbauer 光谱证实了 Fe(IV)2 的氧化态,X 射线晶体学显示 2 具有方形平面配位几何形状以及几种分子内 H⋯C 相互作用。此外,SQUID 磁力计表示室温下的磁矩很小,表明 S = 1 状态可达。密度泛函理论和多构型计算都被用来阐明基态的性质。与实验结果一致,发现基态是 S = 0 态,其中 S = 1 激发态在能量上接近。
C(tBu)Ph)4] (2),产率适中。57 元Fe Mössbauer 光谱证实了 Fe(IV)2 的氧化态,X 射线晶体学显示 2 具有方形平面配位几何形状以及几种分子内 H⋯C 相互作用。此外,SQUID 磁力计表示室温下的磁矩很小,表明 S = 1 状态可达。密度泛函理论和多构型计算都被用来阐明基态的性质。与实验结果一致,发现基态是 S = 0 态,其中 S = 1 激发态在能量上接近。
更新日期:2024-09-10
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph) with FeIICl2 results in isolation of [Li(Et2O)]2[FeII(N
C(tBu)Ph) with FeIICl2 results in isolation of [Li(Et2O)]2[FeII(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph)4] (1), in good yields. The reaction of 1 with 1 equiv. of I2 leads to formation of [FeIV(N
C(tBu)Ph)4] (1), in good yields. The reaction of 1 with 1 equiv. of I2 leads to formation of [FeIV(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(tBu)Ph)4] (2), in moderate yields. 57Fe Mössbauer spectroscopy confirms the Fe(IV) oxidation state of 2, and X-ray crystallography reveals that 2 has a square planar coordination geometry along with several intramolecular H⋯C interactions. Furthermore, SQUID magnetometry indicates a small magnetic moment at room temperature, suggestive of an accessible S = 1 state. Both density functional theory and multiconfigurational calculations were done to elucidate the nature of the ground state. Consistent with the experimental results, the ground state was found to be an S = 0 state with an S = 1 excited state close in energy.
C(tBu)Ph)4] (2), in moderate yields. 57Fe Mössbauer spectroscopy confirms the Fe(IV) oxidation state of 2, and X-ray crystallography reveals that 2 has a square planar coordination geometry along with several intramolecular H⋯C interactions. Furthermore, SQUID magnetometry indicates a small magnetic moment at room temperature, suggestive of an accessible S = 1 state. Both density functional theory and multiconfigurational calculations were done to elucidate the nature of the ground state. Consistent with the experimental results, the ground state was found to be an S = 0 state with an S = 1 excited state close in energy.
中文翻译:

具有低位激发态的同源 Fe(IV) 酮类络合物
4 当量的 Li(N

































 京公网安备 11010802027423号
京公网安备 11010802027423号