当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis, Crystal Structures, and Stereoisomerism of Substituted o,m,o,p-Tetraphenylenes
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.orglett.4c02712 Yuya Kawai 1 , Tomohiro Oriki 1 , Yu Sato 1 , Juntaro Nogami 1 , Yoshinobu Kamiya 1 , Shunsuke Suzuki 1 , Ken Tanaka 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.orglett.4c02712 Yuya Kawai 1 , Tomohiro Oriki 1 , Yu Sato 1 , Juntaro Nogami 1 , Yoshinobu Kamiya 1 , Shunsuke Suzuki 1 , Ken Tanaka 1
Affiliation
|
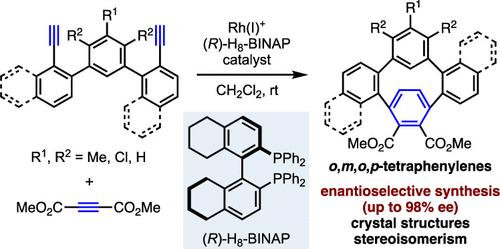
We have achieved the enantioselective synthesis of highly strained, substituted o,m,o,p-tetraphenylenes (≤98% ee) via the cationic Rh(I)/(R)-H8-BINAP complex-catalyzed chemo-, regio-, and enantioselective intermolecular cross-[2+2+2] cycloaddition of teraryl diynes with dimethyl acetylenedicarboxylate. X-ray crystallographic analyses demonstrate the highly bent structures of the para-substituted benzene moieties, and density functional theory calculations reveal the large local strain of the paraphenylene unit. 1H nuclear magnetic resonance analyses and theoretical calculations elucidate the stereoisomerism, indicating that the nonrotatable ortho-disubstituted biphenyl structure results in cis and trans isomers.
中文翻译:

取代的邻、间、邻、对四亚苯基的对映选择性合成、晶体结构和立体异构性
我们通过阳离子Rh(I)/( R )-H 8 -BINAP复合物催化的化学-、区域-实现了高张力、取代的o 、 m 、 o 、 p-四亚苯基(≤98% ee)的对映选择性合成。 ,以及三芳基二炔与乙炔二甲酸二甲酯的对映选择性分子间交叉[2+2+2]环加成。 X射线晶体学分析证明了对位取代苯部分的高度弯曲结构,密度泛函理论计算揭示了对亚苯基单元的大局部应变。 1 H核磁共振分析和理论计算阐明了立体异构现象,表明不可旋转的邻位二取代联苯结构导致顺式和反式异构体。
更新日期:2024-09-10
中文翻译:

取代的邻、间、邻、对四亚苯基的对映选择性合成、晶体结构和立体异构性
我们通过阳离子Rh(I)/( R )-H 8 -BINAP复合物催化的化学-、区域-实现了高张力、取代的o 、 m 、 o 、 p-四亚苯基(≤98% ee)的对映选择性合成。 ,以及三芳基二炔与乙炔二甲酸二甲酯的对映选择性分子间交叉[2+2+2]环加成。 X射线晶体学分析证明了对位取代苯部分的高度弯曲结构,密度泛函理论计算揭示了对亚苯基单元的大局部应变。 1 H核磁共振分析和理论计算阐明了立体异构现象,表明不可旋转的邻位二取代联苯结构导致顺式和反式异构体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号