当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of TYRA-300: First Oral Selective FGFR3 Inhibitor for the Treatment of Urothelial Cancers and Achondroplasia
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-11 , DOI: 10.1021/acs.jmedchem.4c01531 Robert L Hudkins 1 , Eric Allen 1 , Alexandra Balcer 1 , Isaac D Hoffman 1 , Samhita Iyer 1 , Melissa Neal 1 , Kirk J Nelson 1 , Marc Rideout 1 , Qing Ye 1 , Jacqueline H Starrett 1 , Piyush Patel 1 , Todd Harris 1 , Ronald V Swanson 1 , Daniel C Bensen 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-11 , DOI: 10.1021/acs.jmedchem.4c01531 Robert L Hudkins 1 , Eric Allen 1 , Alexandra Balcer 1 , Isaac D Hoffman 1 , Samhita Iyer 1 , Melissa Neal 1 , Kirk J Nelson 1 , Marc Rideout 1 , Qing Ye 1 , Jacqueline H Starrett 1 , Piyush Patel 1 , Todd Harris 1 , Ronald V Swanson 1 , Daniel C Bensen 1
Affiliation
|
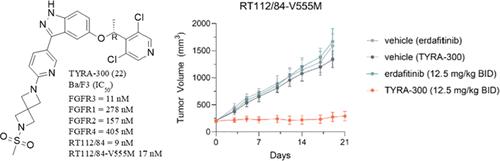
Activating FGFR3 alterations have been identified in up to 15–20% of muscle-invasive bladder cancer and metastatic urothelial carcinoma (mUC), and as high as 80% in nonmuscle invasive bladder cancers. FGFR3 germline mutations have also been associated with a variety of skeletal dysplasias. Achondroplasia, the most common form of dwarfism in humans, results from a G380R mutation in FGFR3. The pan-FGFR inhibitor erdafitinib was approved for the treatment of mUC with FGFR3 alterations but is limited due to FGFR isoform off-target toxicities and the development of on-target gatekeeper resistance mutations. TYRA-300 (22) was conceived using a structure-based approach as a potent FGFR3-selective inhibitor to avoid the toxicities associated with inhibition of FGFR1, FGFR2, and FGFR4, and to be agnostic for the FGFR3 gatekeeper mutations. TYRA-300 is being evaluated in a Phase 1 clinical trial in urothelial cancers and solid tumors, with intention to initiate Phase 2 studies in urothelial cancers and achondroplasia.
中文翻译:

TYRA-300 的发现:首个用于治疗尿路上皮癌和软骨发育不全的口服选择性 FGFR3 抑制剂
在高达 15-20% 的肌层浸润性膀胱癌和转移性尿路上皮癌 (mUC) 中已发现激活 FGFR3 改变,在非肌层浸润性膀胱癌中高达 80%。FGFR3 种系突变也与多种骨骼发育不良有关。软骨发育不全是人类最常见的侏儒症,由 FGFR3 中的 G380R 突变引起。泛 FGFR 抑制剂厄达替尼被批准用于治疗具有 FGFR3 改变的 mUC,但由于 FGFR 亚型脱靶毒性和靶向守门人耐药突变的发展而受到限制。TYRA-300 (22) 使用基于结构的方法构想为一种有效的 FGFR3 选择性抑制剂,以避免与抑制 FGFR1、FGFR2 和 FGFR4 相关的毒性,并且与 FGFR3 守门人突变无关。TYRA-300 正在尿路上皮癌和实体瘤的 1 期临床试验中进行评估,旨在启动尿路上皮癌和软骨发育不全的 2 期研究。
更新日期:2024-09-11
中文翻译:

TYRA-300 的发现:首个用于治疗尿路上皮癌和软骨发育不全的口服选择性 FGFR3 抑制剂
在高达 15-20% 的肌层浸润性膀胱癌和转移性尿路上皮癌 (mUC) 中已发现激活 FGFR3 改变,在非肌层浸润性膀胱癌中高达 80%。FGFR3 种系突变也与多种骨骼发育不良有关。软骨发育不全是人类最常见的侏儒症,由 FGFR3 中的 G380R 突变引起。泛 FGFR 抑制剂厄达替尼被批准用于治疗具有 FGFR3 改变的 mUC,但由于 FGFR 亚型脱靶毒性和靶向守门人耐药突变的发展而受到限制。TYRA-300 (22) 使用基于结构的方法构想为一种有效的 FGFR3 选择性抑制剂,以避免与抑制 FGFR1、FGFR2 和 FGFR4 相关的毒性,并且与 FGFR3 守门人突变无关。TYRA-300 正在尿路上皮癌和实体瘤的 1 期临床试验中进行评估,旨在启动尿路上皮癌和软骨发育不全的 2 期研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号