当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into lysophosphatidylserine recognition and Gα12/13-coupling specificity of P2Y10
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2024-09-11 , DOI: 10.1016/j.chembiol.2024.08.005 Han Yin 1 , Nozomi Kamakura 2 , Yu Qian 1 , Manae Tatsumi 2 , Tatsuya Ikuta 2 , Jiale Liang 1 , Zhenmei Xu 1 , Ruixue Xia 1 , Anqi Zhang 1 , Changyou Guo 1 , Asuka Inoue 3 , Yuanzheng He 1
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2024-09-11 , DOI: 10.1016/j.chembiol.2024.08.005 Han Yin 1 , Nozomi Kamakura 2 , Yu Qian 1 , Manae Tatsumi 2 , Tatsuya Ikuta 2 , Jiale Liang 1 , Zhenmei Xu 1 , Ruixue Xia 1 , Anqi Zhang 1 , Changyou Guo 1 , Asuka Inoue 3 , Yuanzheng He 1
Affiliation
|
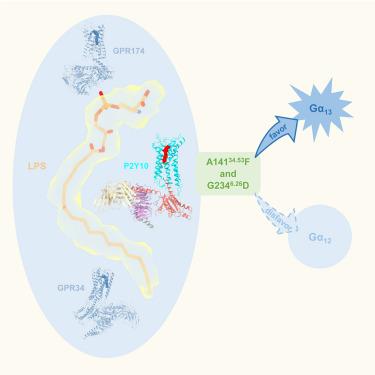
The lysophosphatidylserine (LysoPS) receptor P2Y10, also known as LPS2 , plays crucial roles in the regulation of immune responses and holds promise for the treatment of autoimmune diseases. Here, we report the cryoelectron microscopy (cryo-EM) structure of LysoPS-bound P2Y10 in complex with an engineered G13 heterotrimeric protein. The structure and a mutagenesis study highlight the predominant role of a comprehensive polar network in facilitating the binding and activation of the receptor by LysoPS. This interaction pattern is preserved in GPR174, but not in GPR34. Moreover, our structural study unveils the essential interactions that underlie the Gα13 engagement of P2Y10 and identifies key determinants for Gα12 -vs.-Gα13 -coupling selectivity, whose mutations selectively disrupt Gα12 engagement while preserving the intact coupling of Gα13 . The combined structural and functional studies provide insights into the molecular mechanisms of LysoPS recognition and Gα12/ 13 coupling specificity.
中文翻译:

了解溶血磷脂酰丝氨酸识别和 P2Y10 的 Gα12/13 偶联特异性
溶血磷脂酰丝氨酸 (LysoPS) 受体 P2Y10,也称为 LPS2,在免疫反应的调节中起着至关重要的作用,有望用于治疗自身免疫性疾病。在这里,我们报道了 LysoPS 结合的 P2Y10 与工程化 G13 异源三聚体蛋白复合物的冷冻电子显微镜 (cryo-EM) 结构。结构和诱变研究强调了综合极性网络在促进 LysoPS 结合和激活受体中的主导作用。这种相互作用模式保留在 GPR174 中,但不保留在 GPR34 中。此外,我们的结构研究揭示了 P2Y10 的 Gα13 参与的基本相互作用,并确定了 Gα12 与 Gα13 偶联选择性的关键决定因素,其突变选择性地破坏 Gα12 参与,同时保持 Gα13 的完整偶联。结合结构和功能研究为 LysoPS 识别和 Gα12/13 偶联特异性的分子机制提供了见解。
更新日期:2024-09-11
中文翻译:

了解溶血磷脂酰丝氨酸识别和 P2Y10 的 Gα12/13 偶联特异性
溶血磷脂酰丝氨酸 (LysoPS) 受体 P2Y10,也称为 LPS2,在免疫反应的调节中起着至关重要的作用,有望用于治疗自身免疫性疾病。在这里,我们报道了 LysoPS 结合的 P2Y10 与工程化 G13 异源三聚体蛋白复合物的冷冻电子显微镜 (cryo-EM) 结构。结构和诱变研究强调了综合极性网络在促进 LysoPS 结合和激活受体中的主导作用。这种相互作用模式保留在 GPR174 中,但不保留在 GPR34 中。此外,我们的结构研究揭示了 P2Y10 的 Gα13 参与的基本相互作用,并确定了 Gα12 与 Gα13 偶联选择性的关键决定因素,其突变选择性地破坏 Gα12 参与,同时保持 Gα13 的完整偶联。结合结构和功能研究为 LysoPS 识别和 Gα12/13 偶联特异性的分子机制提供了见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号