当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on Continuous-Flow Process for Direct Synthesis of p-Aminophenol from Nitrobenzene
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-11 , DOI: 10.1021/acs.oprd.4c00275 Jinpeng Huang 1 , Changlu Zhou 1 , Chunping Li 1 , Zhong Xin 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-11 , DOI: 10.1021/acs.oprd.4c00275 Jinpeng Huang 1 , Changlu Zhou 1 , Chunping Li 1 , Zhong Xin 1
Affiliation
|
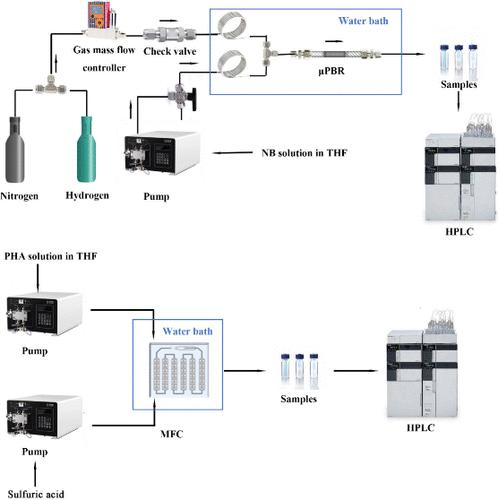
p-Aminophenol (PAP) is an important organic chemical raw material and a pharmaceutical intermediate. Catalytic hydrogenation of nitrobenzene (NB) is an environmentally friendly and economical production method. However, the one-pot method in a traditional batch reactor often leads to a low reaction rate and low PAP yield at low hydrogen pressure. In this work, a continuous-flow process for direct synthesis of PAP by the hydrogenation–rearrangement of NB was established, which provides a safe, green, and efficient method for the synthesis of PAP. The effects of various reaction conditions were investigated. Under the optimal reaction conditions, a 94.5% yield of phenylhydroxylamine (PHA) was achieved in the hydrogenation process under atmospheric pressure. The catalyst activity remained good for 50 h of continuous operation. Solvent tetrahydrofuran (THF) and additive 4-dimethylaminopyridine (DMAP) are more conducive to the synthesis of PHA than other solvents. For different acid catalysts in the Bamberger rearrangement with an equivalent concentration of 2 N, stronger acidity led to greater conversion of PHA. The Bamberger rearrangement is solvent-sensitive, and aprotic solvents will reduce the conversion of PHA. The full continuous process for direct synthesis of PAP from NB was studied by mixing sulfuric acid solution and PHA/THF solution with a microfluidic chip. The conversion of PHA was 100% with a low H2SO4 concentration of 1 wt % at a residence time of 13.6 min. The process was reduced from the hour level of the batch process to the minute level, and the H2SO4 concentration was reduced.
中文翻译:

硝基苯直接合成对氨基苯酚连续流工艺研究
对氨基苯酚(PAP)是一种重要的有机化工原料和医药中间体。硝基苯(NB)催化加氢是一种环保、经济的生产方法。然而,传统间歇式反应器中的一锅法常常导致反应速率低,并且在低氢气压力下PAP产率低。本工作建立了NB加氢重排直接合成PAP的连续流工艺,为PAP的合成提供了一种安全、绿色、高效的方法。研究了各种反应条件的影响。在最佳反应条件下,常压加氢过程中苯羟胺(PHA)收率达到94.5%。连续运行50 h后催化剂活性保持良好。溶剂四氢呋喃(THF)和添加剂4-二甲氨基吡啶(DMAP)比其他溶剂更有利于PHA的合成。对于班伯格重排中不同酸催化剂,当量浓度为2 N时,酸性越强,PHA的转化率越高。 Bamberger 重排对溶剂敏感,非质子溶剂会降低 PHA 的转化。通过微流控芯片将硫酸溶液与PHA/THF溶液混合,研究了从NB直接合成PAP的全连续工艺。在1wt%的低H 2 SO 4浓度和13.6分钟的停留时间下,PHA的转化率为100%。过程由间歇过程的小时级降低到分钟级,H 2 SO 4浓度降低。
更新日期:2024-09-11
中文翻译:

硝基苯直接合成对氨基苯酚连续流工艺研究
对氨基苯酚(PAP)是一种重要的有机化工原料和医药中间体。硝基苯(NB)催化加氢是一种环保、经济的生产方法。然而,传统间歇式反应器中的一锅法常常导致反应速率低,并且在低氢气压力下PAP产率低。本工作建立了NB加氢重排直接合成PAP的连续流工艺,为PAP的合成提供了一种安全、绿色、高效的方法。研究了各种反应条件的影响。在最佳反应条件下,常压加氢过程中苯羟胺(PHA)收率达到94.5%。连续运行50 h后催化剂活性保持良好。溶剂四氢呋喃(THF)和添加剂4-二甲氨基吡啶(DMAP)比其他溶剂更有利于PHA的合成。对于班伯格重排中不同酸催化剂,当量浓度为2 N时,酸性越强,PHA的转化率越高。 Bamberger 重排对溶剂敏感,非质子溶剂会降低 PHA 的转化。通过微流控芯片将硫酸溶液与PHA/THF溶液混合,研究了从NB直接合成PAP的全连续工艺。在1wt%的低H 2 SO 4浓度和13.6分钟的停留时间下,PHA的转化率为100%。过程由间歇过程的小时级降低到分钟级,H 2 SO 4浓度降低。































 京公网安备 11010802027423号
京公网安备 11010802027423号