当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assembly of 2-Substituted Tetrahydroquinolines from ortho-Methylbenzenesulfamides and Dienes, Using a C(sp3)–H Activation/Annulation Sequence
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-11 , DOI: 10.1021/acs.orglett.4c02292 Iván Huertas-Morales 1 , Borja Cendón 1 , Domingo Costa 1 , José Luis Mascareñas 1 , Moisés Gulías 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-11 , DOI: 10.1021/acs.orglett.4c02292 Iván Huertas-Morales 1 , Borja Cendón 1 , Domingo Costa 1 , José Luis Mascareñas 1 , Moisés Gulías 1
Affiliation
|
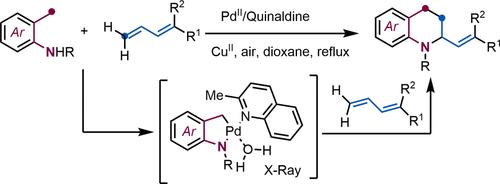
1,2,3,4-Tetrahydroquinolines (THQs) are essential structural cores in many natural products and pharmaceutical drugs. Especially relevant are those presenting substitutions at position 2, yet practical methods for their one-step assembly from acyclic precursors are very scarce. Herein, we present a straightforward approach to assembling these skeletons from ortho-methylanilines using a palladium-catalyzed C(sp3)–H activation/formal cycloaddition sequence. Key for the success of the approach is the use of dienes as partners, since they lead to stable π–allyl palladium intermediates that prevent β-hydride elimination processes and allow installation of versatile alkenyl handles at position 2. Moreover, installing a perfluorobenzenesulfonyl substituent at the amine not only facilitates the C–H activation but also allows for an easy recovery of the free amine.
中文翻译:

使用 C(sp3)–H 激活/成环序列从邻甲基苯磺酰胺和二烯组装 2-取代四氢喹啉
1,2,3,4-四氢喹啉 (THQ) 是许多天然产物和药物的重要结构核心。特别相关的是那些在2位上出现取代的化合物,但从无环前体一步组装它们的实用方法非常稀缺。在此,我们提出了一种使用钯催化的 C(sp 3 )–H 活化/正式环加成序列从邻甲基苯胺组装这些骨架的简单方法。该方法成功的关键是使用二烯作为伙伴,因为它们会产生稳定的π-烯丙基钯中间体,从而防止β-氢化物消除过程并允许在位置2安装通用烯基手柄。此外,在位置安装全氟苯磺酰基取代基胺不仅促进 C-H 活化,而且还可以轻松回收游离胺。
更新日期:2024-09-11
中文翻译:

使用 C(sp3)–H 激活/成环序列从邻甲基苯磺酰胺和二烯组装 2-取代四氢喹啉
1,2,3,4-四氢喹啉 (THQ) 是许多天然产物和药物的重要结构核心。特别相关的是那些在2位上出现取代的化合物,但从无环前体一步组装它们的实用方法非常稀缺。在此,我们提出了一种使用钯催化的 C(sp 3 )–H 活化/正式环加成序列从邻甲基苯胺组装这些骨架的简单方法。该方法成功的关键是使用二烯作为伙伴,因为它们会产生稳定的π-烯丙基钯中间体,从而防止β-氢化物消除过程并允许在位置2安装通用烯基手柄。此外,在位置安装全氟苯磺酰基取代基胺不仅促进 C-H 活化,而且还可以轻松回收游离胺。































 京公网安备 11010802027423号
京公网安备 11010802027423号