当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Direct Asymmetric Aldol Reaction of Glycine Schiff Bases to Access syn-β-Hydroxy-α-amino Esters
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.orglett.4c03085 Toshifumi Takeuchi 1 , Masakatsu Shibasaki 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.orglett.4c03085 Toshifumi Takeuchi 1 , Masakatsu Shibasaki 1
Affiliation
|
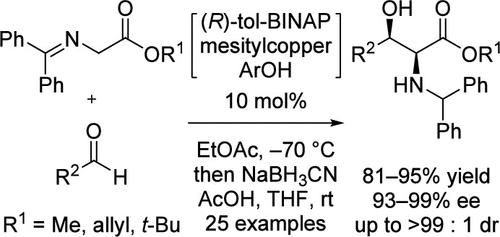
This study reported a copper-catalyzed direct asymmetric aldol reaction between aldehydes and glycine Schiff bases with methyl, allyl, and tert-butyl esters. Additionally, this reaction afforded high yields of syn-β-hydroxy-α-amino esters with excellent enantio- and diastereoselectivities (93–99% ee, up to >99:1 dr). The aldol reaction accepted aromatic, linear aliphatic, and α-substituted aliphatic aldehydes.
中文翻译:

铜催化甘氨酸席夫碱直接不对称羟醛反应生成顺式-β-羟基-α-氨基酯
该研究报道了醛和甘氨酸席夫碱与甲基、烯丙基和叔丁基酯之间的铜催化的直接不对称羟醛反应。此外,该反应提供了高产率的顺式-β-羟基-α-氨基酯,具有优异的对映选择性和非对映选择性(93–99% ee,高达>99:1 dr)。羟醛反应接受芳香族、直链脂肪族和α-取代的脂肪族醛。
更新日期:2024-09-10
中文翻译:

铜催化甘氨酸席夫碱直接不对称羟醛反应生成顺式-β-羟基-α-氨基酯
该研究报道了醛和甘氨酸席夫碱与甲基、烯丙基和叔丁基酯之间的铜催化的直接不对称羟醛反应。此外,该反应提供了高产率的顺式-β-羟基-α-氨基酯,具有优异的对映选择性和非对映选择性(93–99% ee,高达>99:1 dr)。羟醛反应接受芳香族、直链脂肪族和α-取代的脂肪族醛。































 京公网安备 11010802027423号
京公网安备 11010802027423号