当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Progress in Enzyme-Catalyzed C(sp3)–H Amination
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-09-11 , DOI: 10.1021/acscatal.4c04947 Wei-Nan Xu 1 , Ya-Dong Gao 2 , Ping Su 3 , Luqi Huang 3 , Zhao-Lin He 1 , Li-Cheng Yang 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-09-11 , DOI: 10.1021/acscatal.4c04947 Wei-Nan Xu 1 , Ya-Dong Gao 2 , Ping Su 3 , Luqi Huang 3 , Zhao-Lin He 1 , Li-Cheng Yang 2
Affiliation
|
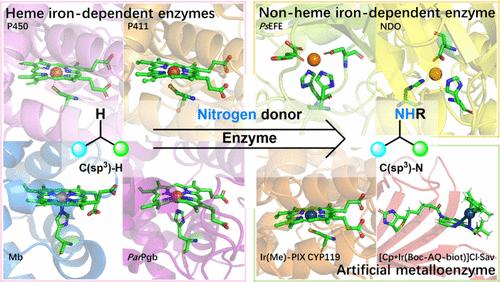
Amine structures are widely present in various biologically active natural products, drug molecules, and material structures. Among the various strategies of amine synthesis, C(sp3)–H amination has become a powerful strategy due to its atom economy and multiple potential reaction sites compared with conventional amine synthesis methods. Due to the advantages of high catalytic efficiency, high selectivity, environmental friendliness, and high modifiability of the enzyme, the enzymatic C(sp3)–H amination is of great research significance. However, it was not until recent years that natural enzymes capable of catalyzing the amination of C(sp3)–H bonds were discovered. Modifying enzymes to confer unnatural C(sp3)–H amination activity holds great potential. In the past decade, a series of protocols for the amination of C(sp3)–H bonds using engineered enzymes have been developed, several of which showed comparable properties to those of natural enzymes.
中文翻译:

酶催化C(sp3)–H胺化研究进展
胺结构广泛存在于各种具有生物活性的天然产物、药物分子和材料结构中。在各种胺合成策略中,与传统胺合成方法相比,C(sp 3 )–H胺化由于其原子经济性和多个潜在反应位点而成为一种强大的策略。由于酶催化效率高、选择性高、环境友好、可修饰性强等优点,酶促C(sp 3 )–H胺化具有重要的研究意义。然而,直到最近几年,才发现能够催化C(sp 3 )–H键胺化的天然酶。修饰酶以赋予非自然的 C(sp 3 )–H 胺化活性具有巨大的潜力。在过去的十年中,已经开发了一系列使用工程酶对 C(sp 3 )–H 键进行胺化的方案,其中一些方案显示出与天然酶相当的特性。
更新日期:2024-09-11
中文翻译:

酶催化C(sp3)–H胺化研究进展
胺结构广泛存在于各种具有生物活性的天然产物、药物分子和材料结构中。在各种胺合成策略中,与传统胺合成方法相比,C(sp 3 )–H胺化由于其原子经济性和多个潜在反应位点而成为一种强大的策略。由于酶催化效率高、选择性高、环境友好、可修饰性强等优点,酶促C(sp 3 )–H胺化具有重要的研究意义。然而,直到最近几年,才发现能够催化C(sp 3 )–H键胺化的天然酶。修饰酶以赋予非自然的 C(sp 3 )–H 胺化活性具有巨大的潜力。在过去的十年中,已经开发了一系列使用工程酶对 C(sp 3 )–H 键进行胺化的方案,其中一些方案显示出与天然酶相当的特性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号