当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental observation of molecular-weight growth by the reactions of o-benzyne with benzyl radicals
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4cp02560a David E Couch 1, 2 , Myrsini M San Marchi 2 , Nils Hansen 2
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4cp02560a David E Couch 1, 2 , Myrsini M San Marchi 2 , Nils Hansen 2
Affiliation
|
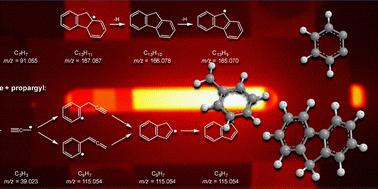
The chemistry of ortho-benzyne (o-C6H4) is of fundamental importance due to its role as an essential molecular building block in molecular-weight growth reactions. Here, we report on an experimental investigation of the reaction of o-C6H4 with benzyl (C7H7) radicals in a well-controlled flash pyrolysis experiment using a resistively heated SiC microtubular reactor at temperatures of 800–1600 K and pressures near 30 torr. To this end, the reactants o-C6H4 and C7H7 were pyrolytically generated from 1,2-diiodobenzene and benzyl bromide, respectively. Using molecular-beam time-of-flight mass spectrometry, we found that o-C6H4 associates with the benzyl to form C13H11 radicals, which decompose at higher temperatures via H-loss to form closed-shell C13H10 molecules. Our experimental results agree with earlier theoretical calculations by Matsugi and Miyoshi [Phys. Chem. Chem. Phys., 2012, 14, 9722–9728], who predicted the formation of fluorene (C13H10) + H to be the dominant reaction channel. At temperatures above 1400 K, we also observed the formation of C13H9 radicals, most likely the resonance-stabilized fluorenyl π-radical. Our study confirms that molecular-mass growth via the o-C6H4 + C7H7 reaction provides a versatile pathway for introducing five-membered rings, and hence curved structures, into polycyclic aromatic hydrocarbons.
中文翻译:

邻苄炔与苄基自由基反应分子量增长的实验观察
邻苯苄 (o-C 6H4) 的化学性质具有至关重要性,因为它在分子量生长反应中起着重要的分子结构单元的作用。在这里,我们报告了在 800-1600 K 的温度和接近 30 torr 的压力下,使用电阻加热的 SiC 微管反应器在控制良好的闪蒸热解实验中 o-C 6H4 与苄基 (C7H7) 自由基的反应的实验研究。为此,分别由 1,2-二碘苯和溴苄热解生成反应物 o-C 6H4 和 C7H7。使用分子束飞行时间质谱法,我们发现 o-C 6H4 与苄基结合形成 C13H11 自由基,这些自由基在较高温度下通过 H-loss 分解形成闭壳层 C13H10 分子。我们的实验结果与 Matsugi 和 Miyoshi [Phys. Chem. Chem. Phys., 2012, 14, 9722–9728] 的早期理论计算一致,他们预测芴 (C13H10) + H 的形成是主要的反应通道。在高于 1400 K 的温度下,我们还观察到 C13H9 自由基的形成,很可能是共振稳定的芴基 π 自由基。 我们的研究证实,通过 o-C 6H4 + C7H7 反应的分子量增长为将五元环和弯曲结构引入多环芳烃提供了一种通用途径。
更新日期:2024-09-11
中文翻译:

邻苄炔与苄基自由基反应分子量增长的实验观察
邻苯苄 (o-C 6H4) 的化学性质具有至关重要性,因为它在分子量生长反应中起着重要的分子结构单元的作用。在这里,我们报告了在 800-1600 K 的温度和接近 30 torr 的压力下,使用电阻加热的 SiC 微管反应器在控制良好的闪蒸热解实验中 o-C 6H4 与苄基 (C7H7) 自由基的反应的实验研究。为此,分别由 1,2-二碘苯和溴苄热解生成反应物 o-C 6H4 和 C7H7。使用分子束飞行时间质谱法,我们发现 o-C 6H4 与苄基结合形成 C13H11 自由基,这些自由基在较高温度下通过 H-loss 分解形成闭壳层 C13H10 分子。我们的实验结果与 Matsugi 和 Miyoshi [Phys. Chem. Chem. Phys., 2012, 14, 9722–9728] 的早期理论计算一致,他们预测芴 (C13H10) + H 的形成是主要的反应通道。在高于 1400 K 的温度下,我们还观察到 C13H9 自由基的形成,很可能是共振稳定的芴基 π 自由基。 我们的研究证实,通过 o-C 6H4 + C7H7 反应的分子量增长为将五元环和弯曲结构引入多环芳烃提供了一种通用途径。













































 京公网安备 11010802027423号
京公网安备 11010802027423号