Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic immunotherapy with a calcium-based nanoinducer: evoking pyroptosis and remodeling tumor-associated macrophages for enhanced antitumor immune response
Nanoscale ( IF 5.8 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4nr01497a Fang Cheng 1 , Lei He 1 , Jiaqi Wang 1 , Lunhui Lai 1 , Li Ma 1 , Kuiming Qu 1 , Zicheng Yang 1 , Xinyue Wang 1 , Ruyu Zhao 1 , Lixing Weng 1 , Lianhui Wang 1
Nanoscale ( IF 5.8 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4nr01497a Fang Cheng 1 , Lei He 1 , Jiaqi Wang 1 , Lunhui Lai 1 , Li Ma 1 , Kuiming Qu 1 , Zicheng Yang 1 , Xinyue Wang 1 , Ruyu Zhao 1 , Lixing Weng 1 , Lianhui Wang 1
Affiliation
|
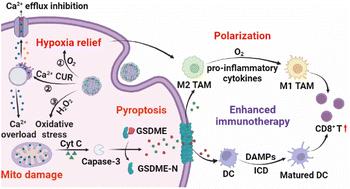
The challenges posed by low immunogenicity and the immunosuppressive tumor microenvironment (TME) significantly hinder the efficacy of cancer immunotherapy. Pyroptosis, characterized as a pro-inflammatory cell death pathway, emerges as a promising approach to augment immunotherapy by promoting immunogenic cell death (ICD). The predominance of M2 phenotype tumor-associated macrophages (TAMs) in the TME underscores the critical need for TAM reprogramming to mitigate this immunosuppression. Herein, we introduce a calcium-based, intelligent-responsive nanoinducer (CaZCH NPs), designed to concurrently initiate pyroptosis and remodel TAMs, thereby amplifying antitumor immunotherapy effects. Modified with hyaluronic acid, CaZCH NPs can target tumor cells. Once internalized, CaZCH NPs respond to the acidic environment, releasing Ca2+, curcumin and H2O2 to induce mitochondrial Ca2+ overload and oxidation stress, leading to caspase-3/GSDME-mediated cell pyroptosis. Concurrently, O2 produced by CaZCH and pro-inflammatory cytokines from pyroptotic cells work together to shift TAM polarization towards the M1 phenotype, effectively countering TME's immunosuppressive effect. Notably, the synergistic effect of Ca2+-mediated pyroptosis and TAM remodeling demonstrates superior antitumor efficiency in colorectal cancer models. The induced ICD, coupled with M1-type TAMs, effectively enhances immunogenicity and mitigates immunosuppression, promoting dendritic cell maturation and activating CD8+ T cell-dependent systemic antitumor immunity. Our study presents a promising synergistic strategy for achieving highly efficient immunotherapy using a simple calcium-based nanoinducer.
中文翻译:

基于钙的纳米诱导剂的协同免疫疗法:诱发细胞焦亡并重塑肿瘤相关巨噬细胞以增强抗肿瘤免疫反应
低免疫原性和免疫抑制性肿瘤微环境 (TME) 带来的挑战严重阻碍了癌症免疫疗法的疗效。焦亡 (Pyroptosis) 的特征是促炎细胞死亡途径,是一种通过促进免疫原性细胞死亡 (ICD) 来增强免疫治疗的有前途的方法。M2 表型肿瘤相关巨噬细胞 (TAM) 在 TME 中占主导地位,这凸显了 TAM 重编程以减轻这种免疫抑制的迫切需求。在此,我们介绍了一种基于钙的智能响应纳米诱导剂 (CaZCH NPs),旨在同时启动细胞焦亡和重塑 TAM,从而放大抗肿瘤免疫治疗效果。用透明质酸修饰的 CaZCH NPs 可以靶向肿瘤细胞。一旦内化,CaZCH NPs 对酸性环境做出反应,释放 Ca2+、姜黄素和 H2O2 以诱导线粒体 Ca2+ 过载和氧化应激,导致 caspase-3/GSDME 介导的细胞焦亡。同时,CaZCH 产生的 O2 和焦亡细胞的促炎细胞因子共同作用,将 TAM 极化转向 M1 表型,有效对抗 TME 的免疫抑制作用。值得注意的是,Ca2+ 介导的焦亡和 TAM 重塑的协同作用在结直肠癌模型中表现出卓越的抗肿瘤效率。诱导的 ICD 与 M1 型 TAMs 偶联,有效增强免疫原性并减轻免疫抑制,促进树突状细胞成熟并激活 CD8+ T 细胞依赖性全身抗肿瘤免疫。 我们的研究提出了一种有前途的协同策略,可以使用简单的钙基纳米诱导剂实现高效的免疫治疗。
更新日期:2024-09-11
中文翻译:

基于钙的纳米诱导剂的协同免疫疗法:诱发细胞焦亡并重塑肿瘤相关巨噬细胞以增强抗肿瘤免疫反应
低免疫原性和免疫抑制性肿瘤微环境 (TME) 带来的挑战严重阻碍了癌症免疫疗法的疗效。焦亡 (Pyroptosis) 的特征是促炎细胞死亡途径,是一种通过促进免疫原性细胞死亡 (ICD) 来增强免疫治疗的有前途的方法。M2 表型肿瘤相关巨噬细胞 (TAM) 在 TME 中占主导地位,这凸显了 TAM 重编程以减轻这种免疫抑制的迫切需求。在此,我们介绍了一种基于钙的智能响应纳米诱导剂 (CaZCH NPs),旨在同时启动细胞焦亡和重塑 TAM,从而放大抗肿瘤免疫治疗效果。用透明质酸修饰的 CaZCH NPs 可以靶向肿瘤细胞。一旦内化,CaZCH NPs 对酸性环境做出反应,释放 Ca2+、姜黄素和 H2O2 以诱导线粒体 Ca2+ 过载和氧化应激,导致 caspase-3/GSDME 介导的细胞焦亡。同时,CaZCH 产生的 O2 和焦亡细胞的促炎细胞因子共同作用,将 TAM 极化转向 M1 表型,有效对抗 TME 的免疫抑制作用。值得注意的是,Ca2+ 介导的焦亡和 TAM 重塑的协同作用在结直肠癌模型中表现出卓越的抗肿瘤效率。诱导的 ICD 与 M1 型 TAMs 偶联,有效增强免疫原性并减轻免疫抑制,促进树突状细胞成熟并激活 CD8+ T 细胞依赖性全身抗肿瘤免疫。 我们的研究提出了一种有前途的协同策略,可以使用简单的钙基纳米诱导剂实现高效的免疫治疗。































 京公网安备 11010802027423号
京公网安备 11010802027423号