Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering macrophage membrane-camouflaged nanoplatforms with enhanced macrophage function for mediating sonodynamic therapy of ovarian cancer
Nanoscale ( IF 5.8 ) Pub Date : 2024-09-10 , DOI: 10.1039/d4nr01307g Xiaofei Wang , Hongling Wang , Yansheng Li , Zhihong Sun , Jie Liu , Xiaoli Cao , Chengming Sun
Nanoscale ( IF 5.8 ) Pub Date : 2024-09-10 , DOI: 10.1039/d4nr01307g Xiaofei Wang , Hongling Wang , Yansheng Li , Zhihong Sun , Jie Liu , Xiaoli Cao , Chengming Sun
|
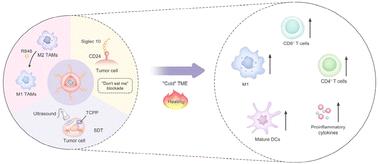
Cancer immunotherapy has demonstrated remarkable efficacy in the treatment of cancer, and it has been successfully applied in the treatment of various solid tumors. However, the response rates to immunotherapy in patients with ovarian cancer remain modest because of the immunosuppressive tumor microenvironment (TME). Tumor-associated macrophages (TAMs) represent the predominant myeloid cell population within the TME, which adopt the protumorigenic M2 phenotype and are blinded by the “don't eat me” signals from tumor cells. These characteristics of TAMs result in insufficient phagocytic activation. In this study, we constructed a SIM@TR-NP-mediated combination therapy of sonodynamic and immunotherapy. SIM@TR-NPs were modified by engineered macrophage membranes with overexpressed sialic acid-binding Ig-like lectin 10 (Siglec-10), and were internally loaded with sonosensitizer 4,4′,4′′,4′′′-(porphine-5,10,15,20-tetrayl)tetrakis(benzoic acid) and immune adjuvant resiquimod. SIM@TR-NPs can block “don't eat me” signals to enhance macrophage phagocytosis and trigger the polarization of TAMs toward the M1 phenotype, thereby improving the immunosuppressive TME. Simultaneously, upon ultrasound irradiation, SIM@TR-NP-mediated sonodynamic therapy (SDT) triggered immunogenic cell death in tumor cells, in combination with TAM-based immunotherapy, transforming the “immune cold tumor” into an “immune hot tumor”. SIM@TR-NP-mediated sonodynamic immunotherapy exhibited potent antitumor efficacy in ovarian cancer and exhibited substantial potential for improving the immunosuppressive TME. This study presents an emerging therapeutic regimen for ovarian cancer that synergizes TAM-based antitumor immunotherapy and SDT.
中文翻译:

工程化巨噬细胞膜伪装纳米平台,增强巨噬细胞功能,介导卵巢癌的声动力学治疗
癌症免疫疗法在癌症治疗中显示出显著的疗效,并已成功应用于各种实体瘤的治疗。然而,由于免疫抑制性肿瘤微环境 (TME),卵巢癌患者对免疫治疗的反应率仍然适中。肿瘤相关巨噬细胞 (TAM) 代表 TME 中的主要髓系细胞群,它们采用促瘤性 M2 表型,并被来自肿瘤细胞的“不要吃我”信号所蒙蔽。TAM 的这些特性导致吞噬激活不足。在这项研究中,我们构建了一种 SIM@TR-NP 介导的声动力学和免疫疗法联合疗法。SIM@TR-NPs 被过表达唾液酸结合 Ig 样凝集素 10 (Siglec-10) 的工程巨噬细胞膜修饰,并在内部加载声敏剂 4,4',4'′,4′′-(卟啉-5,10,15,20-四基)四基(苯甲酸)和免疫佐剂 resiquimod。SIM@TR-NPs 可以阻断“don't eat me”信号,以增强巨噬细胞吞噬作用并触发 TAMs 向 M1 表型的极化,从而改善免疫抑制 TME。同时,在超声照射下,SIM@TR-NP 介导的声动力学疗法 (SDT) 触发肿瘤细胞免疫原性细胞死亡,结合基于 TAM 的免疫疗法,将“免疫性冷肿瘤”转化为“免疫性热肿瘤”。SIM@TR-NP 介导的声动力学免疫疗法在卵巢癌中表现出强大的抗肿瘤功效,并显示出改善免疫抑制 TME 的巨大潜力。本研究提出了一种新兴的卵巢癌治疗方案,该方案将基于 TAM 的抗肿瘤免疫疗法和 SDT 协同作用。
更新日期:2024-09-10
中文翻译:

工程化巨噬细胞膜伪装纳米平台,增强巨噬细胞功能,介导卵巢癌的声动力学治疗
癌症免疫疗法在癌症治疗中显示出显著的疗效,并已成功应用于各种实体瘤的治疗。然而,由于免疫抑制性肿瘤微环境 (TME),卵巢癌患者对免疫治疗的反应率仍然适中。肿瘤相关巨噬细胞 (TAM) 代表 TME 中的主要髓系细胞群,它们采用促瘤性 M2 表型,并被来自肿瘤细胞的“不要吃我”信号所蒙蔽。TAM 的这些特性导致吞噬激活不足。在这项研究中,我们构建了一种 SIM@TR-NP 介导的声动力学和免疫疗法联合疗法。SIM@TR-NPs 被过表达唾液酸结合 Ig 样凝集素 10 (Siglec-10) 的工程巨噬细胞膜修饰,并在内部加载声敏剂 4,4',4'′,4′′-(卟啉-5,10,15,20-四基)四基(苯甲酸)和免疫佐剂 resiquimod。SIM@TR-NPs 可以阻断“don't eat me”信号,以增强巨噬细胞吞噬作用并触发 TAMs 向 M1 表型的极化,从而改善免疫抑制 TME。同时,在超声照射下,SIM@TR-NP 介导的声动力学疗法 (SDT) 触发肿瘤细胞免疫原性细胞死亡,结合基于 TAM 的免疫疗法,将“免疫性冷肿瘤”转化为“免疫性热肿瘤”。SIM@TR-NP 介导的声动力学免疫疗法在卵巢癌中表现出强大的抗肿瘤功效,并显示出改善免疫抑制 TME 的巨大潜力。本研究提出了一种新兴的卵巢癌治疗方案,该方案将基于 TAM 的抗肿瘤免疫疗法和 SDT 协同作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号