Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multimodal imaging of a liver-on-a-chip model using labelled and label-free optical microscopy techniques
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4lc00504j Jan Majer 1, 2 , Aneesh Alex 3, 4 , Jindou Shi 4, 5, 6 , Eric J Chaney 4, 5 , Prabuddha Mukherjee 4, 5 , Darold R Spillman 4, 5, 7 , Marina Marjanovic 4, 5, 7, 8 , Carla F Newman 1 , Reid M Groseclose 3 , Peter D Watson 2 , Stephen A Boppart 4, 5, 6, 7, 8, 9 , Steve R Hood 1, 4
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4lc00504j Jan Majer 1, 2 , Aneesh Alex 3, 4 , Jindou Shi 4, 5, 6 , Eric J Chaney 4, 5 , Prabuddha Mukherjee 4, 5 , Darold R Spillman 4, 5, 7 , Marina Marjanovic 4, 5, 7, 8 , Carla F Newman 1 , Reid M Groseclose 3 , Peter D Watson 2 , Stephen A Boppart 4, 5, 6, 7, 8, 9 , Steve R Hood 1, 4
Affiliation
|
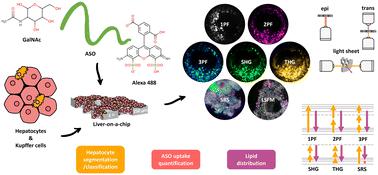
A liver-on-a-chip model is an advanced complex in vitro model (CIVM) that incorporates different cell types and extracellular matrix to mimic the microenvironment of the human liver in a laboratory setting. Given the heterogenous and complex nature of liver-on-a-chip models, brightfield and fluorescence-based imaging techniques are widely utilized for assessing the changes occurring in these models with different treatment and environmental conditions. However, the utilization of optical microscopy techniques for structural and functional evaluation of the liver CIVMs have been limited by the reduced light penetration depth and lack of 3D information obtained using these imaging techniques. In this study, the potential of both labelled as well as label-free multimodal optical imaging techniques for visualization and characterization of the cellular and sub-cellular features of a liver-on-a-chip model was investigated. (1) Cellular uptake and distribution of Alexa 488 (A488)-labelled non-targeted and targeted antisense oligonucleotides (ASO and ASO-GalNAc) in the liver-on-a-chip model was determined using multiphoton microscopy. (2) Hyperspectral stimulated Raman scattering (SRS) microscopy of the C–H region was used to determine the heterogeneity of chemical composition of circular and cuboidal hepatocytes in the liver-on-a-chip model in a label-free manner. Additionally, the spatial overlap between the intracellular localization of ASO and lipid droplets was explored using simultaneous hyperspectral SRS and fluorescence microscopy. (3) The capability of light sheet fluorescence microscopy (LSFM) for full-depth 3D visualization of sub-cellular distribution of A488-ASO and cellular phenotypes in the liver-on-a-chip model was demonstrated. In summary, multimodal optical microscopy is a promising platform that can be utilized for visualization and quantification of 3D cellular organization, drug distribution and functional changes occurring in liver-on-a-chip models, and can provide valuable insights into liver biology and drug uptake mechanisms by enabling better characterization of these liver models.
中文翻译:

使用标记和无标记光学显微镜技术对肝脏芯片模型进行多模态成像
肝脏芯片模型是一种先进的复杂体外模型 (CIVM),它结合了不同的细胞类型和细胞外基质,以在实验室环境中模拟人类肝脏的微环境。鉴于肝脏芯片模型的异质性和复杂性,基于明场和荧光的成像技术被广泛用于评估这些模型在不同治疗和环境条件下发生的变化。然而,利用光学显微镜技术对肝脏 CIVM 进行结构和功能评估受到光穿透深度减少和缺乏使用这些成像技术获得的 3D 信息的限制。在这项研究中,研究了标记和无标记多模态光学成像技术在肝脏芯片模型的细胞和亚细胞特征的可视化和表征方面的潜力。 (1) 使用多光子显微镜测定肝脏芯片模型中 Alexa 488 (A488) 标记的非靶向和靶向反义寡核苷酸(ASO 和 ASO-GalNAc)的细胞摄取和分布。 (2)使用C-H区的高光谱受激拉曼散射(SRS)显微镜以无标记的方式确定肝脏芯片模型中圆形和立方形肝细胞化学成分的异质性。此外,使用同步高光谱 SRS 和荧光显微镜探索了 ASO 和脂滴的细胞内定位之间的空间重叠。 (3) 展示了光片荧光显微镜 (LSFM) 对 A488-ASO 的亚细胞分布和肝脏芯片模型中的细胞表型进行全深度 3D 可视化的能力。 总之,多模态光学显微镜是一个有前途的平台,可用于肝脏芯片模型中发生的 3D 细胞组织、药物分布和功能变化的可视化和量化,并可以为肝脏生物学和药物摄取提供有价值的见解通过更好地表征这些肝脏模型来研究机制。
更新日期:2024-09-11
中文翻译:

使用标记和无标记光学显微镜技术对肝脏芯片模型进行多模态成像
肝脏芯片模型是一种先进的复杂体外模型 (CIVM),它结合了不同的细胞类型和细胞外基质,以在实验室环境中模拟人类肝脏的微环境。鉴于肝脏芯片模型的异质性和复杂性,基于明场和荧光的成像技术被广泛用于评估这些模型在不同治疗和环境条件下发生的变化。然而,利用光学显微镜技术对肝脏 CIVM 进行结构和功能评估受到光穿透深度减少和缺乏使用这些成像技术获得的 3D 信息的限制。在这项研究中,研究了标记和无标记多模态光学成像技术在肝脏芯片模型的细胞和亚细胞特征的可视化和表征方面的潜力。 (1) 使用多光子显微镜测定肝脏芯片模型中 Alexa 488 (A488) 标记的非靶向和靶向反义寡核苷酸(ASO 和 ASO-GalNAc)的细胞摄取和分布。 (2)使用C-H区的高光谱受激拉曼散射(SRS)显微镜以无标记的方式确定肝脏芯片模型中圆形和立方形肝细胞化学成分的异质性。此外,使用同步高光谱 SRS 和荧光显微镜探索了 ASO 和脂滴的细胞内定位之间的空间重叠。 (3) 展示了光片荧光显微镜 (LSFM) 对 A488-ASO 的亚细胞分布和肝脏芯片模型中的细胞表型进行全深度 3D 可视化的能力。 总之,多模态光学显微镜是一个有前途的平台,可用于肝脏芯片模型中发生的 3D 细胞组织、药物分布和功能变化的可视化和量化,并可以为肝脏生物学和药物摄取提供有价值的见解通过更好地表征这些肝脏模型来研究机制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号