Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A nanoporous hydrogel-based model to study chemokine gradient-driven angiogenesis under luminal flow
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4lc00460d Nidhi Mote , Sarah Kubik , William Polacheck , Brendon Baker , Britta Trappmann
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4lc00460d Nidhi Mote , Sarah Kubik , William Polacheck , Brendon Baker , Britta Trappmann
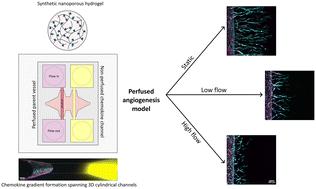
|
The growth of new blood vessels through angiogenesis is a highly coordinated process, which is initiated by chemokine gradients that activate endothelial cells within a perfused parent vessel to sprout into the surrounding 3D tissue matrix. While both biochemical signals from pro-angiogenic factors, as well as mechanical cues originating from luminal fluid flow that exerts shear stress on the vessel wall, have individually been identified as major regulators of endothelial cell sprouting, it remains unclear whether and how both types of cues synergize. To fill this knowledge gap, here, we created a 3D biomimetic model of chemokine gradient-driven angiogenic sprouting, in which a micromolded tube inside a hydrogel matrix is seeded with endothelial cells and connected to a perfusion system to control fluid flow rates and resulting shear forces on the vessel wall. To allow for the formation of chemokine gradients despite the presence of luminal flow, a nanoporous synthetic hydrogel that supports angiogenesis but limits the interstitial flow proved crucial. Using this system, we find that luminal flow and resulting shear stress is a major regulator of the speed and morphogenesis of angiogenic sprouting, whose action is mediated through changes in vascular permeability.
中文翻译:

一种基于纳米多孔水凝胶的模型,用于研究管腔血流下趋化因子梯度驱动的血管生成
通过血管生成新血管的生长是一个高度协调的过程,该过程由趋化因子梯度启动,趋化因子梯度激活灌注母血管内的内皮细胞,发芽到周围的 3D 组织基质中。虽然来自促血管生成因子的生化信号,以及源自对血管壁施加剪切应力的管腔液流动的机械信号,都已被单独确定为内皮细胞发芽的主要调节因子,但尚不清楚这两种类型的信号是否以及如何协同作用。为了填补这一知识空白,我们在这里创建了一个趋化因子梯度驱动的血管生成发芽的 3D 仿生模型,其中水凝胶基质内的微成型管接种了内皮细胞并连接到灌注系统,以控制流体流速和产生的对血管壁的剪切力。为了在存在管腔血流的情况下允许趋化因子梯度的形成,支持血管生成但限制间质血流的纳米多孔合成水凝胶被证明是至关重要的。使用该系统,我们发现管腔流动和由此产生的剪切应力是血管生成发芽速度和形态发生的主要调节因子,其作用是通过血管通透性的变化介导的。
更新日期:2024-09-11
中文翻译:

一种基于纳米多孔水凝胶的模型,用于研究管腔血流下趋化因子梯度驱动的血管生成
通过血管生成新血管的生长是一个高度协调的过程,该过程由趋化因子梯度启动,趋化因子梯度激活灌注母血管内的内皮细胞,发芽到周围的 3D 组织基质中。虽然来自促血管生成因子的生化信号,以及源自对血管壁施加剪切应力的管腔液流动的机械信号,都已被单独确定为内皮细胞发芽的主要调节因子,但尚不清楚这两种类型的信号是否以及如何协同作用。为了填补这一知识空白,我们在这里创建了一个趋化因子梯度驱动的血管生成发芽的 3D 仿生模型,其中水凝胶基质内的微成型管接种了内皮细胞并连接到灌注系统,以控制流体流速和产生的对血管壁的剪切力。为了在存在管腔血流的情况下允许趋化因子梯度的形成,支持血管生成但限制间质血流的纳米多孔合成水凝胶被证明是至关重要的。使用该系统,我们发现管腔流动和由此产生的剪切应力是血管生成发芽速度和形态发生的主要调节因子,其作用是通过血管通透性的变化介导的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号