当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An insight into separating H2 from natural gas/H2 mixtures using Mg-based systems
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4ta05654j Mateusz Balcerzak 1, 2, 3 , Robert Urbanczyk 1, 4 , Fabian Lange 1 , Francis Anne Helm 1 , Jan Ternieden 1 , Michael Felderhoff 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4ta05654j Mateusz Balcerzak 1, 2, 3 , Robert Urbanczyk 1, 4 , Fabian Lange 1 , Francis Anne Helm 1 , Jan Ternieden 1 , Michael Felderhoff 1
Affiliation
|
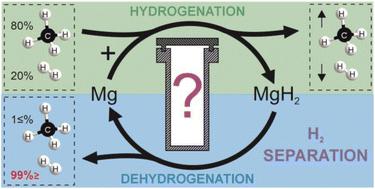
The use of light, abundant, and relatively cheap Mg-based systems arouses great interest in hydrogen-economy-related applications such as hydrogen and heat storage. So far, MgH2, capable of storing large amounts of H2 (7.6 wt%), has been scarcely evaluated for its H2 separation potential, which may be crucial for H2 recovery from various H2-containing gas mixtures. Herein, we reveal and discuss the ability of Mg-based systems to separate H2 from CH4-rich gas mixtures. Mg-Ni and Mg-Fe systems can separate ∼5.5 wt% of H2 during the hydrogenation process and release pure H2 (at least 99.9%) within the dehydrogenation process. Pure H2 can, therefore, be obtained in a one-step separation system. In this study, we discuss the selection of the hydrogenation/dehydrogenation processes catalyst (Ni, Fe) as well as the optimal separation process temperature. The tested systems show satisfactory performance stability during cyclic H2 separation from CH4/H2 and natural gas/H2 gas mixtures. We also present the first investigation of the Mg-based systems (with Ni or Fe catalyst) after the cycled separation processes. The results of complementary techniques revealed H2 separation-induced chemical and phase segregation in the studied materials. Moreover, we report the observation of networked MgH2 microstructure formation. This research points out the potential of metal-hydrides in the H2 separation sector as well as the challenges facing their application – especially those related to the presence of CO2 impurity in the gas mixture. The unique and detailed description of processes taking place in a reactor during the separation process will significantly impact the design of future metal-hydrides-based scaled-up systems for H2 separation.
中文翻译:

深入了解使用镁基系统从天然气/氢气混合物中分离氢气
轻质、丰富且相对便宜的镁基系统的使用引起了人们对氢经济相关应用(例如氢和储热)的极大兴趣。迄今为止,能够储存大量H 2 (7.6wt%)的MgH 2几乎没有对其H 2分离潜力进行评估,这对于从各种含H 2气体混合物中回收H 2可能是至关重要的。在此,我们揭示并讨论了镁基系统从富含CH 4的气体混合物中分离H 2的能力。 Mg-Ni和Mg-Fe系统可以在加氢过程中分离~5.5wt%的H 2 ,并在脱氢过程中释放纯H 2 (至少99.9%)。因此,可以在一步分离系统中获得纯H 2 。在本研究中,我们讨论了加氢/脱氢过程催化剂(Ni、Fe)的选择以及最佳分离过程温度。测试的系统在从CH 4 /H 2和天然气/H 2气体混合物中循环分离H 2期间表现出令人满意的性能稳定性。我们还首次研究了循环分离过程后的镁基系统(使用镍或铁催化剂)。 互补技术的结果揭示了所研究材料中 H 2分离引起的化学和相分离。此外,我们报告了网络状 MgH 2微观结构形成的观察结果。这项研究指出了金属氢化物在 H 2分离领域的潜力及其应用所面临的挑战,尤其是与气体混合物中存在 CO 2杂质相关的挑战。对分离过程中反应器中发生的过程的独特而详细的描述将显着影响未来基于金属氢化物的放大的H 2分离系统的设计。
更新日期:2024-09-11
中文翻译:

深入了解使用镁基系统从天然气/氢气混合物中分离氢气
轻质、丰富且相对便宜的镁基系统的使用引起了人们对氢经济相关应用(例如氢和储热)的极大兴趣。迄今为止,能够储存大量H 2 (7.6wt%)的MgH 2几乎没有对其H 2分离潜力进行评估,这对于从各种含H 2气体混合物中回收H 2可能是至关重要的。在此,我们揭示并讨论了镁基系统从富含CH 4的气体混合物中分离H 2的能力。 Mg-Ni和Mg-Fe系统可以在加氢过程中分离~5.5wt%的H 2 ,并在脱氢过程中释放纯H 2 (至少99.9%)。因此,可以在一步分离系统中获得纯H 2 。在本研究中,我们讨论了加氢/脱氢过程催化剂(Ni、Fe)的选择以及最佳分离过程温度。测试的系统在从CH 4 /H 2和天然气/H 2气体混合物中循环分离H 2期间表现出令人满意的性能稳定性。我们还首次研究了循环分离过程后的镁基系统(使用镍或铁催化剂)。 互补技术的结果揭示了所研究材料中 H 2分离引起的化学和相分离。此外,我们报告了网络状 MgH 2微观结构形成的观察结果。这项研究指出了金属氢化物在 H 2分离领域的潜力及其应用所面临的挑战,尤其是与气体混合物中存在 CO 2杂质相关的挑战。对分离过程中反应器中发生的过程的独特而详细的描述将显着影响未来基于金属氢化物的放大的H 2分离系统的设计。































 京公网安备 11010802027423号
京公网安备 11010802027423号