当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ construction of dual-functional Ni/NixB catalysts for the hydrogenation and dehydrogenation of magnesium hydride
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4ta05395h Hui Liang 1 , Wenjiang Li 2 , Jie Zheng 3
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4ta05395h Hui Liang 1 , Wenjiang Li 2 , Jie Zheng 3
Affiliation
|
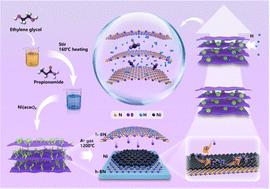
Hydrogen sorption catalysts loaded on porous supports can promote reversible H ↔ H0 and metal ↔ metal ion reactions. However, uniform dispersion of catalytic nanoparticles in the matrix is required to synergistically promote MgH2 hydrogenation, diffusion, and dehydrogenation. In this study, we decorated hexagonal boron nitride (h-BN) in situ with NixB. Experimental characterization demonstrated the uniform distribution of NixB in the h-BN matrix. The nickel atoms were introduced into the porous h-BN matrix through Ni–B bonds, which effectively limited nanoparticle growth. Simulations of the hydrogenation and dehydrogenation pathways of several Ni/NixB@MgH2 composites suggested that the Ni/NixB catalyst should have efficient catalytic performance. The composite had a hydrogen storage capacity of approximately 7.0 wt% at 200 °C and released approximately 4.5 wt% H2 within 10 min, with rapid kinetics and stable reversible cycling. Finally, the thermodynamics and kinetics of hydrogenation/dehydrogenation in the presence of the composite catalysts were theoretically evaluated.
中文翻译:

原位构建用于氢化镁加氢和脱氢的双功能Ni/NixB催化剂
负载在多孔载体上的氢吸附催化剂可以促进可逆的H↔H 0和金属↔金属离子反应。然而,需要催化纳米粒子在基质中均匀分散以协同促进MgH 2加氢、扩散和脱氢。在本研究中,我们用 Ni x B原位修饰六方氮化硼 (h-BN)。实验表征表明 Ni x B 在 h-BN 基体中均匀分布。镍原子通过Ni-B键引入多孔h-BN基体中,有效限制了纳米粒子的生长。对几种Ni/Ni x B@MgH 2复合材料的加氢和脱氢路径的模拟表明Ni/Ni x B催化剂应具有高效的催化性能。该复合材料在200℃下具有约7.0wt%的储氢能力,并在10分钟内释放约4.5wt%的H 2 ,具有快速的动力学和稳定的可逆循环。最后,对复合催化剂存在下的加氢/脱氢热力学和动力学进行了理论评估。
更新日期:2024-09-11
中文翻译:

原位构建用于氢化镁加氢和脱氢的双功能Ni/NixB催化剂
负载在多孔载体上的氢吸附催化剂可以促进可逆的H↔H 0和金属↔金属离子反应。然而,需要催化纳米粒子在基质中均匀分散以协同促进MgH 2加氢、扩散和脱氢。在本研究中,我们用 Ni x B原位修饰六方氮化硼 (h-BN)。实验表征表明 Ni x B 在 h-BN 基体中均匀分布。镍原子通过Ni-B键引入多孔h-BN基体中,有效限制了纳米粒子的生长。对几种Ni/Ni x B@MgH 2复合材料的加氢和脱氢路径的模拟表明Ni/Ni x B催化剂应具有高效的催化性能。该复合材料在200℃下具有约7.0wt%的储氢能力,并在10分钟内释放约4.5wt%的H 2 ,具有快速的动力学和稳定的可逆循环。最后,对复合催化剂存在下的加氢/脱氢热力学和动力学进行了理论评估。































 京公网安备 11010802027423号
京公网安备 11010802027423号