当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly selective catalytic oxidation of methane to methanol using Cu–Pd/anatase
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4ee02671c Liqun Wang 1 , Jingting Jin 1 , Wenzhi Li 1, 2 , Cunshuo Li 1 , Leyu Zhu 1 , Zheng Zhou 1 , Lulu Zhang 3 , Xia Zhang 1 , Liang Yuan 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-09-11 , DOI: 10.1039/d4ee02671c Liqun Wang 1 , Jingting Jin 1 , Wenzhi Li 1, 2 , Cunshuo Li 1 , Leyu Zhu 1 , Zheng Zhou 1 , Lulu Zhang 3 , Xia Zhang 1 , Liang Yuan 4
Affiliation
|
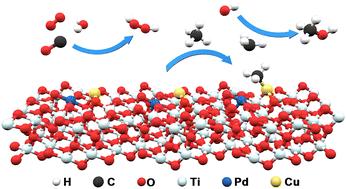
Direct conversion of methane into high value-added products is of great practical significance. The synergistic effect in catalysts with dual-active components show potential to increase the methanol yield and selectivity. In this work, Cu–Pd/anatase is in situ generated and exhibits a relatively high methanol yield rate of ∼31 800 μmol gcat−1 h−1 and near-exclusive selectivity of liquid products (methanol). The reaction mechanism behind the heterogeneous catalysis process has been investigated. It is confirmed that copper ions hold the ability to produce hydrogen peroxide which can be further promoted by anatase. Chlorine ions can promote the stable adsorption of CO and the formation of *CH3 intermediates, facilitating high activity and selectivity for methanol production. Pd and Cu cooperatively dissociate methane, which promotes the formation of key configuration metal-CH3. The ˙CH3 intermediate desorption will be facilitated on Cu–Pd/anatase through the manner of electron regulation, which is proved by the combination of density functional theory calculations and in situ infrared spectroscopy. Methanol is formed when a ˙CH3 is desorbed from a copper site and combines with a hydroxyl radical.
中文翻译:

使用 Cu-Pd/锐钛矿将甲烷高选择性催化氧化为甲醇
将甲烷直接转化为高附加值产品具有重要的现实意义。双活性组分催化剂的协同效应显示出提高甲醇产率和选择性的潜力。在这项工作中,Cu-Pd/锐钛矿是原位生成的,并表现出相对较高的甲醇产率(∼31 800 μmol g cat -1 h -1)和液体产物(甲醇)的近乎独有的选择性。研究了多相催化过程背后的反应机理。已证实铜离子具有产生过氧化氢的能力,锐钛矿可进一步促进这种能力。氯离子可以促进CO的稳定吸附和*CH 3中间体的形成,有利于甲醇生产的高活性和选择性。 Pd和Cu协同解离甲烷,促进关键构型金属-CH 3的形成。密度泛函理论计算和原位红外光谱相结合证明了Cu-Pd/锐钛矿上通过电子调控的方式促进了CH 3中间体的解吸。当 ˙CH 3从铜位点解吸并与羟基自由基结合时,会形成甲醇。
更新日期:2024-09-11
中文翻译:

使用 Cu-Pd/锐钛矿将甲烷高选择性催化氧化为甲醇
将甲烷直接转化为高附加值产品具有重要的现实意义。双活性组分催化剂的协同效应显示出提高甲醇产率和选择性的潜力。在这项工作中,Cu-Pd/锐钛矿是原位生成的,并表现出相对较高的甲醇产率(∼31 800 μmol g cat -1 h -1)和液体产物(甲醇)的近乎独有的选择性。研究了多相催化过程背后的反应机理。已证实铜离子具有产生过氧化氢的能力,锐钛矿可进一步促进这种能力。氯离子可以促进CO的稳定吸附和*CH 3中间体的形成,有利于甲醇生产的高活性和选择性。 Pd和Cu协同解离甲烷,促进关键构型金属-CH 3的形成。密度泛函理论计算和原位红外光谱相结合证明了Cu-Pd/锐钛矿上通过电子调控的方式促进了CH 3中间体的解吸。当 ˙CH 3从铜位点解吸并与羟基自由基结合时,会形成甲醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号