Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Novel Cu(II), Co(II), Fe(II), and Ni(II) Hydrazone Metal Complexes as Potent Anticancer Agents: Spectroscopic, DFT, Molecular Docking, and MD Simulation Studies
ACS Omega ( IF 3.7 ) Pub Date : 2024-09-10 , DOI: 10.1021/acsomega.4c06202 Eyüp Basaran 1 , Hatice Gamze Sogukomerogullari 2 , Muhammed Tılahun Muhammed 3 , Senem Akkoc 4, 5
ACS Omega ( IF 3.7 ) Pub Date : 2024-09-10 , DOI: 10.1021/acsomega.4c06202 Eyüp Basaran 1 , Hatice Gamze Sogukomerogullari 2 , Muhammed Tılahun Muhammed 3 , Senem Akkoc 4, 5
Affiliation
|
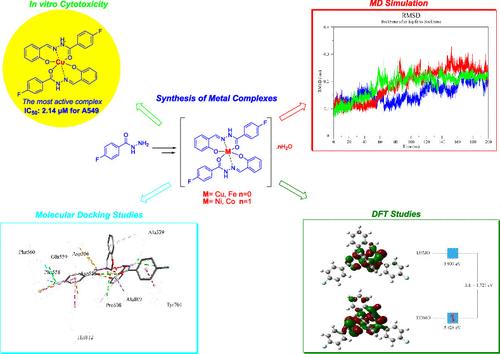
Metal complexes [FeL], [NiL]·H2O, [CuL], and [CoL]·H2O were formed by the ligand (L, 4-fluoro-N′-(2-hydroxybenzylidene)benzohydrazide) reacting with Fe(OAc)2, Ni(OAc)2·4H2O, Cu(OAc)2·H2O, and Co(OAc)2·4H2O. The produced compounds were characterized using a variety of methods, such as NMR, UV–vis, FT-IR, magnetic susceptibility, elemental analysis, and molar conductivity. The spectrum of the data indicates that the geometry of the complex molecular structures is octahedral with six coordination sites. The ligand and its different metal complexes were tested in a human lung cancer cell line and a normal embryonic kidney cell line. A cytotoxic assay revealed that L-Cu is the most potent chelate against cancer cell lines. A computational study was performed to rationalize this finding. The binding potential of relatively active compounds to a suitable target was analyzed. For this purpose, a target that is known to be inhibited by small compounds with a scaffold similar to that of the synthesized compounds, lysine-specific demethylase 1 (LSD1), was first determined. Molecular docking studies demonstrated that L-Cu has a high binding potential to LSD1 at a level comparable to that of a standard ligand. Molecular dynamics (MD) simulations revealed that L-Cu and L form stable complexes with the enzyme. Furthermore, the MD simulation study showed that L-Cu remained inside the binding pocket of the enzyme during the 200 ns simulation period. Density functional theory (DFT) studies demonstrated that the chemical stability of L was higher than that of its chelate form, L-Cu.
中文翻译:

新型 Cu(II)、Co(II)、Fe(II) 和 Ni(II) 腙金属配合物的合成作为有效的抗癌剂:光谱、DFT、分子对接和 MD 模拟研究
金属络合物 [FeL], [NiL]·H2O、[CuL] 和 [CoL]·配体(L,4-氟-N′-(2-羟基亚苄基)苯并酰肼)与Fe(OAc)2、Ni(OAc)2·4H2O、Cu(OAc)2·H2O 和 Co(OAc)2·4H2O。使用多种方法对产生的化合物进行表征,例如 NMR、UV-Vis、FT-IR、磁化率、元素分析和摩尔电导率。数据范围表明,复杂分子结构的几何形状是八面体,具有六个配位点。配体及其不同的金属复合物在人肺癌细胞系和正常胚胎肾细胞系中进行了测试。细胞毒性测定显示,L-Cu 是针对癌细胞系最有效的螯合物。进行了一项计算研究以合理化这一发现。分析了相对活性化合物与合适靶标的结合潜力。为此,首先确定了已知被小化合物抑制的靶标,其支架与合成化合物的支架相似,即赖氨酸特异性脱甲基酶 1 (LSD1)。分子对接研究表明,L-Cu 与 LSD1 具有很高的结合电位,其水平与标准配体相当。分子动力学 (MD) 模拟表明 L-Cu 和 L 与酶形成稳定的复合物。此外,MD 模拟研究表明,在 200 ns 模拟期间,L-Cu 保持在酶的结合口袋内。密度泛函理论 (DFT) 研究表明,L 的化学稳定性高于其螯合物形式 L-Cu。
更新日期:2024-09-10
中文翻译:

新型 Cu(II)、Co(II)、Fe(II) 和 Ni(II) 腙金属配合物的合成作为有效的抗癌剂:光谱、DFT、分子对接和 MD 模拟研究
金属络合物 [FeL], [NiL]·H2O、[CuL] 和 [CoL]·配体(L,4-氟-N′-(2-羟基亚苄基)苯并酰肼)与Fe(OAc)2、Ni(OAc)2·4H2O、Cu(OAc)2·H2O 和 Co(OAc)2·4H2O。使用多种方法对产生的化合物进行表征,例如 NMR、UV-Vis、FT-IR、磁化率、元素分析和摩尔电导率。数据范围表明,复杂分子结构的几何形状是八面体,具有六个配位点。配体及其不同的金属复合物在人肺癌细胞系和正常胚胎肾细胞系中进行了测试。细胞毒性测定显示,L-Cu 是针对癌细胞系最有效的螯合物。进行了一项计算研究以合理化这一发现。分析了相对活性化合物与合适靶标的结合潜力。为此,首先确定了已知被小化合物抑制的靶标,其支架与合成化合物的支架相似,即赖氨酸特异性脱甲基酶 1 (LSD1)。分子对接研究表明,L-Cu 与 LSD1 具有很高的结合电位,其水平与标准配体相当。分子动力学 (MD) 模拟表明 L-Cu 和 L 与酶形成稳定的复合物。此外,MD 模拟研究表明,在 200 ns 模拟期间,L-Cu 保持在酶的结合口袋内。密度泛函理论 (DFT) 研究表明,L 的化学稳定性高于其螯合物形式 L-Cu。





























 京公网安备 11010802027423号
京公网安备 11010802027423号