当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Electrophilic Allenoates [and Isocyanates/Isothiocyanates] with a 2-Alkynylpyridine via a Free Carbene Intermediate
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.orglett.4c02387 Ruiqin Wang 1 , Qian Xu 1 , Thomas R Hoye 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.orglett.4c02387 Ruiqin Wang 1 , Qian Xu 1 , Thomas R Hoye 1
Affiliation
|
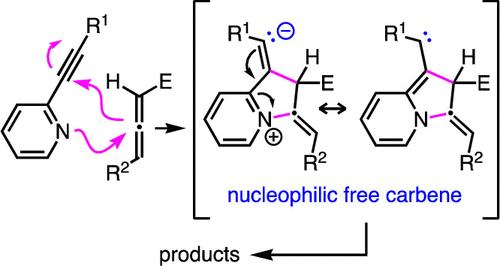
A pyridine containing a 2-alkynyl substituent armed with a carbene reporter group [R1 = C(Me)2OAc] is shown to engage electrophilic allenes to generate intermediate free carbenes. Depending on the electron density at the carbene carbon atom, a feature that is modulated by the substituents on the allene substrate, the carbene will either rearrange or eject an acetate leaving group, leading to various types of indolizine-containing products. Iso(thio)cyanates react in an analogous fashion.
中文翻译:

亲电联烯酸酯[和异氰酸酯/异硫氰酸酯]与 2-炔基吡啶通过游离卡宾中间体的反应
含有带有卡宾报告基团 [R 1 = C(Me) 2 OAc] 的 2-炔基取代基的吡啶被证明可以与亲电子丙二烯结合生成中间体游离卡宾。根据卡宾碳原子上的电子密度(由丙二烯底物上的取代基调节的特征),卡宾将重新排列或排出乙酸酯离去基团,从而产生各种类型的含中氮茚产物。异(硫)氰酸酯以类似的方式发生反应。
更新日期:2024-09-10
中文翻译:

亲电联烯酸酯[和异氰酸酯/异硫氰酸酯]与 2-炔基吡啶通过游离卡宾中间体的反应
含有带有卡宾报告基团 [R 1 = C(Me) 2 OAc] 的 2-炔基取代基的吡啶被证明可以与亲电子丙二烯结合生成中间体游离卡宾。根据卡宾碳原子上的电子密度(由丙二烯底物上的取代基调节的特征),卡宾将重新排列或排出乙酸酯离去基团,从而产生各种类型的含中氮茚产物。异(硫)氰酸酯以类似的方式发生反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号